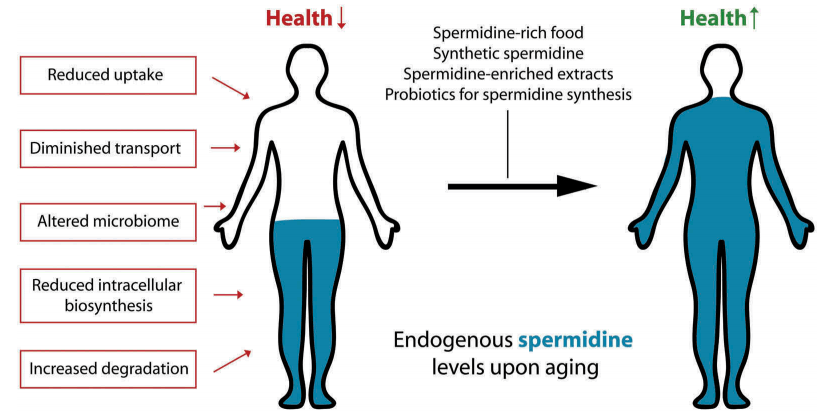
A physiological autophagy inducer acting as an anti-aging vitamin in humans?
Spermidine is a natural polyamine that stimulates cytoprotective macroautophagy/autophagy. External supplementation of spermidine extends lifespan and health span across species, including in yeast, nematodes, flies and mice. In humans, spermidine levels decline with aging, and a possible connection between reduced endogenous spermidine concentrations and age-related deterioration has been suggested. Recent epidemiological data support this notion, showing that an increased uptake of this polyamine with spermidine-rich food diminishes overall mortality associated with cardiovascular diseases and cancer. Here, we discuss nutritional and other possible routes to counteract the age-mediated decline of spermidine levels.
Spermidine is a natural polyamine present in all living organisms that is critically involved in the maintenance of cellular homeostasis. This chemical affects numerous biological processes, including cell growth and proliferation, tissue regeneration, DNA and RNA stabilization, enzymatic modulation, and regulation of translation, among others . Furthermore, spermidine exhibits anti-inflammatory and antioxidant properties, enhances mitochondrial metabolic function and respiration, promotes chaperone activity and improves proteostasis . Intriguingly, external supplementation of spermidine exerts various beneficial effects on aging and age-related disease in a variety of model organisms, including mice . For example, spermidine feeding extends lifespan across species , promotes cardio- and neuroprotection, stimulates antineoplastic immune response and may avoid immunosenescence by stimulating memory T-cell formation . Many of these antiaging properties have been causally linked to the capacity of spermidine to ensure proteostasis through the stimulation of cytoprotective macroautophagy [15–17]. Age-associated conditions including cancer, neurodegeneration and cardiovascular diseases are directly connected to the intracellular accumulation of toxic debris, and its removal by autophagy constitutes a well-documented avenue for protection against age and disease.
which is mainly driven by acetylation and subsequent oxidation [29–31], but also on its uptake from the extracellular space and its excretion by the cell. This may be mediated by membrane transporters such as the ones operating in yeast and bacteria , but could also involve endocytosis/exocytosis processes. Still, polyamine transport in mammals remains not well understood and needs further examination.The intestinal microbiota represents another source of spermidine synthesis within our body. In mice, the concentration of spermidine in the gut lumen has been shown to directly depend on the colonic microbiota [33]. Thus, commensal gut bacteria may regulate polyamine concentration in the human intestine [34]. In fact, preclinical studies support the critical involvement of the gut microbiome in generating health-relevant spermidine. Therefore, blood levels of spermidine and spermine can be upregulated through oral administration of the polyamine-producing probiotic Bifidobacterium LKM512, resulting in suppressed inflammation and improved longevity in old mice [35]. Interestingly, these effects can be enhanced, if combined with additional supplementation of arginine, a polyamine precursor [36]. Thus, arginine may be considered as a prebiotic for stimulating intestinal spermidine synthesis. However, other prebiotic strategies may consist of providing agents that favor the selective expansion of polyamine-producing bacteria or the upregulation of spermidine synthesis by the existing gut microbiota. Dietary spermidine is rapidly resorbed from the intestine and distributed in the body without degradation [37].
Within mammalian cells, spermidine is generated from its precursor putrescine (which itself is generated from ornithine) or by oxidative degradation of spermine. The net cytosolic spermidine content further depends on its catabolism,
Dietary spermidine is rapidly resorbed from the intestine and distributed in the body without degradation [37]. Thus, food items with high spermidine content can contribute to raising the bioavailability of this polyamine. Such spermidinerich food items comprise unprocessed plant-derived foods including the durian fruit, shitake mushrooms, fresh green pepper, wheat germ, amaranth grain, cauliflower and broccoli, just to mention a few, but also products resulting from fermentation processes that involve polyamine-generating bacteria and fungi, e.g. soybean products such as natto or many types of mature cheese [38]. Therefore, the systemic levels of spermidine are influenced by diet either directly, by the ingestion of polyamine-rich food items, or indirectly, by effects of the diet on the spermidine-producing microbiota. The use of a spermidine-rich diet to elevate systemic spermidine levels in human organs [39] may constitute a promising strategy for promoting healthy aging. Spermidine shows no adverse effects during life-long administration in mice [5], and a currently ongoing clinical trial on supplementation of spermidine-rich plant extracts to elderly persons indicates good safety and tolerability [40].
Dietary spermidine is rapidly resorbed from the intestine and distributed in the body without degradation [37]. Thus, food items with high spermidine content can contribute to raising the bioavailability of this polyamine. Such spermidinerich food items comprise unprocessed plant-derived foods including the durian fruit, shitake mushrooms, fresh green pepper, wheat germ, amaranth grain, cauliflower and broccoli, just to mention a few, but also products resulting from fermentation processes that involve polyamine-generating bacteria and fungi, e.g. soybean products such as natto or many types of mature cheese [38]. Therefore, the systemic levels of spermidine are influenced by diet either directly, by the ingestion of polyamine-rich food items, or indirectly, by effects of the diet on the spermidine-producing microbiota. The use of a spermidine-rich diet to elevate systemic spermidine levels in human organs [39] may constitute a promising strategy for promoting healthy aging. Spermidine shows no adverse effects during life-long administration in mice [5], and a currently ongoing clinical trial on supplementation of spermidine-rich plant extracts to elderly persons indicates good safety and tolerability [40].
In sum, in our view, spermidine is synthesized by our organism in sufficient quantities during youth, but not in old age. Thus, one may argue that, as we age, spermidine evolves to the status of a vitamin, and thus has to be supplemented from external sources to secure the maintenance of autophagic flux required for organismal homeostasis. Conflict of interest GK, FM and DC-G are scientific co-founders of Samsara Therapeutics. FM and DC-G have equity interest in The Longevity Labs. GK is a cofounder of everImmune. Disclosure statement GK, FM and DC-G are scientific co-founders of Samsara Therapeutics. FM and DC-G have equity interest in The Longevity Labs. GK is a cofounder of everImmune.
Funding
GK is supported by the Ligue contre le Cancer (équipe labellisée); Agence National de la Recherche (ANR) – Projets blancs; ANR under the frame of E-Rare-2, the ERA-Net for Research on Rare Diseases; Association pour la recherche sur le cancer (ARC); Cancéropôle Ile-de-France; Chancelerie des universités de Paris (Legs Poix), Fondation pour la Recherche Médicale (FRM); a donation by Elior; the European Commission (ArtForce); European Research Area Network on Cardiovascular Diseases (ERACVD, MINOTAUR); the European Research Council (ERC); Fondation Carrefour; Institut National du Cancer (INCa); Inserm (HTE); Institut Universitaire de France; LeDucq Foundation; the LabEx ImmunoOncology; the RHU Torino Lumière; the Seerave Foundation; the SIRIC Stratified Oncology Cell DNA Repair and Tumor Immune Elimination (SOCRATE); the SIRIC Cancer Research and Personalized Medicine (CARPEM); and the Paris Alliance of Cancer Research Institutes (PACRI). F.M. is grateful to the Austrian Science Fund FWF (Austria) for grants P23490-B20, P29262, P24381, P29203 P27893, I1000, “SFB Lipotox” (F3012), and DKplus Metabolic and Cardiovascular Diseases (W1226), as well as to Bundesministerium für Wissenschaft, Forschung und Wirtschaft and the Karl-Franzens University for grants“Unkonventionelle Forschung”. We acknowledge support from NAWI Graz and the BioTechMed-Graz flagship project “EPIAge.”.