Spermine and gene methylation: a mechanism of lifespan extension induced by polyamine‑rich diet
The polyamines spermidine and spermine are synthesized in almost all organisms and are also contained in food. Polyamine synthesis decreases with aging, but no signifcant decrease in polyamine concentrations were found in organs, tissues, and blood of adult animals and humans. We found that healthy dietary patterns were associated with a preference for polyaminerich foods, and frst reported that increased polyamine intake extended the lifespan of mice and decreased the incidence of colon cancer induced by repeated administration of moderate amounts of a carcinogen. Recent investigations have revealed that changes in DNA methylation status play an important role in lifespan and aging-associated pathologies. The methylation of DNA is regulated by DNA methyltransferases in the presence of S-adenosylmethionine. Decarboxylated S-adenosylmethionine, converted from S-adenosylmethionine by S-adenosylmethionine decarboxylase, provides an aminopropyl group to synthesize spermine and spermidine and acts to inhibit DNMT activity. Long-term increased polyamine intake were shown to elevate blood spermine levels in mice and humans. In vitro studies demonstrated that spermine reversed changes induced by the inhibition of ornithine decarboxylase (e.g., increased decarboxylated S-adenosylmethionine, decreased DNA methyltransferase activity, increased aberrant DNA methylation), whose activity decreases with aging. Further, aged mice fed high-polyamine chow demonstrated suppression of aberrant DNA methylation and a consequent increase in protein levels of lymphocyte function-associated antigen 1, which plays a pivotal role on infammatory process.
This review discusses the relation between polyamine metabolism and DNA methylation, as well as the biological mechanism of lifespan extension induced by increased polyamine intake.
Introduction
Polyamines, as represented by spermine, spermidine, and their precursor putrescine, are indispensable for cell growth and diferentiation. They have many biological activities, such as gene expression, transcription, cellular signaling, and protein synthesis, and they act to protect cells and genes from harmful stimuli. We found that polyamines suppress the synthesis of proinfammatory cytokines and decrease cell adhesion by selectively decreasing lymphocyte function-associated antigen 1 (LFA-1), which is involved in immune cell activation and infammation. The anti-infammatory properties of polyamines are not accompanied by a decreased cellular activity. Polyamines maintain biological activities and extend lifespan of immune cells taken from human blood and cultured in plastic plate (Soda et al. 2005). Chronic infammation and the resulting increase in oxidative stress have been shown to contribute to most aging-associated chronic diseases. Moreover, aging itself is associated with a proinfammatory status, e.g., immune system dysregulation leading to chronic mild infammation and sustained oxidative stress (Soda et al. 2005).Based on the benefcial functions of polyamines and the fact that they are derived primarily from food, we started to examine the possible role of dietary polyamines in maintaining a long, healthy life. We frst found that chow with added synthetic polyamines extended the life span of mice (Soda et al. 2009a).
Substances contained in foods that inhibit or counteract the aging-associated proinfammatory status and decrease resulting increases in oxidative stress (i.e., chemicals that inhibit the transfer of electrons from a substance to an oxidizing agent) on lifespan extension have been attracted scientists’ interest. Among these substances, antioxidant polyphenols and antioxidant vitamins are considered to be important candidates for extending healthy lifespans. Examples include isofavones, found at high levels in soybeans, and resveratrol and vitamins, which are prevalent in the Mediterranean diet. The molecules have many biological activities that may counteract the pathogenesis of aging-associated pathologies. For example, they have antioxidant and anti-infammatory properties, and they activate autophagy (Ferraresi et al. 2017; Sacks et al. 2006; Wang et al. 2018b; Zhang et al. 2018) and protect cells and genes from harmful stimuli (George et al. 2017; Guthrie et al. 2017). However, many studies have failed to show any efects on the prevention of aging-associated pathologies and the extension of lifespan (Burnett et al. 2011; Sacks et al. 2006; Staats et al. 2018; Strong et al. 2013). Polyamines also have biological activities similar to those of antioxidant polyphenols and vitamins; for instance, they exert anti-infammatory (Paul and Kang 2013; Soda et al. 2005) and antioxidant properties (Rider et al. 2007), protect cells and genes from harmful stimuli (Douki et al. 2000; Okumura et al. 2016; Pothipongsa et al. 2012), and promote autophagy (Sacitharan et al. 2018).
However, in the case of antioxidant polyphenols and vitamins, these functions do not extend the lifespan in mammals, so they are unlikely to play the major role in the extension of mouse lifespans by polyamines. Here, I discuss the biological process whereby increased polyamine intake extends humans and animals lifespans, and refer to previously published studies to summarize the efects of aging on polyamine concentrations in the body and the efects of increased polyamine intake on polyamine concentrations.
Aging and polyamine concentration
The activities of enzymes involved in polyamine synthesis, especially ornithine decarboxylase (ODC), decrease with aging (Yoshinaga et al. 1993). And, age-dependent decreases in polyamine concentrations in tissues, organs, blood, and urine have been reported in animals and humans. In the older papers published before 1985, it was reported that spermidine and spermine concentrations in tissues and organs in rats decreased with aging, and their concentrations in brain and muscles of older rats were lower than those in adult animals (Das and Kanungo 1982; Jaenne et al. 1964). However, there was as signifcant decline in spermine and spermidine concentrations during young age, and this decline seemed to slow markedly by adulthood. In the recent articles, the descriptions in abstracts and titles indicated that aging-associated declines in polyamine concentrations occurs during early life. Nishimura et al.found that polyamine concentrations in various tissues and organs were signifcantly lower in 10- and 26-week old mice than in 3-week-old mice, but no diferences in spermine and spermidine concentrations were observed between 10- and 26-week-old mice, except skin (Nishimura et al. 2006).
Polyamine concentrations in blood cells, especially erythrocytes and leukocytes, and urinary polyamine excretion are known to refect polyamine levels in organs and tissues throughout the body. In human blood, no age-associated decline in spermine or spermidine concentrations were observed, but large inter-individual diferences were found (Elworthy and Hitchcock 1989; Soda et al. 2005). Elworthy and Hitchcock measured red blood cell polyamine concentrations in 117 patients (ranging from 0 to 80 years old) who were largely in good health but had various neurological problems known not to afect polyamine levels. No statistically signifcant age-dependent changes in spermine or spermidine concentrations was observed (Elworthy and Hitchcock 1989). Our three separate analyses of aging-associated changes in blood polyamine concentrations in human volunteers [42 males whose age raged from 26 to 69 (Soda et al. 2005), 58 males ranging from 40 to 69 years old (Soda et al. unpublished), and 33 females from 61 to 83 years old (Soda et al. unpublished)] showed similar fndings, namely no decrease in blood spermidine or spermine concentrations.The blood samples were examined by two diferent laboratories, and the results were the same. Madeo et al. stated (2018) that we reported an age-dependent decline in spermidine concentrations in human organs (Soda et al. 2009b), but our manuscript contained no text, tables, or fgures indicating an age-dependent decline in spermidine concentrations in the organs of either humans or animals.
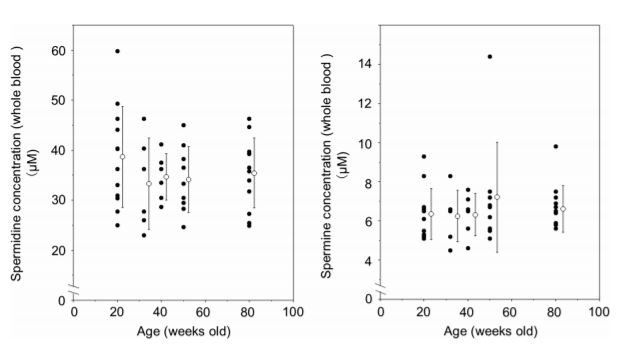
I summarize blood polyamine concentrations in three separate mouse experiments published previously (Soda et al. 2009a, b, 2013), and there was no aging-associated changes in blood polyamine concentrations in mice fed chow of which polyamine concentrations were not increased (Fig. 1).Similarly, urinary polyamine excretion, which refects blood polyamine concentrations, does not change with age during adulthood. van den Berg et al. measured urinary polyamine excretion in 51 healthy volunteers whose ages ranged from 4 days to 77 years, and found an age-dependent decrease in urinary excretion of spermidine in terms of creatinine excretion. However, they clearly indicated that the overall age-dependent decline was merely due to the rapid decrease during the frst year of life, and it did not occur during adulthood (van den Berg et al. 1986). Yodfat also examined urinary polyamine concentrations in 171 male and 166 female healthy volunteers whose ages ranged from 14 days to 84 years (Yodfat et al. 1988). They demonstrated an age-dependent decrease in diamine levels in male, but no age-dependent decrease of polyamines (either spermidine or spermine) in either gender. These data indicate that fndings of age-associated decrease in polyamine concentrations refect their rapid decline during early life, and no decrease is observed in healthy adult animals and humans.
Dietary polyamine and epidemiological studies
Foods contain polyamines, though at widely varying levels (Cipolla et al. 2007; Nishibori et al. 2006; Nishimura et al. 2006; Soda et al. 2017).
Therefore, personal food preferences and regional dietary patterns may greatly afect polyamine intake from food. We examined the relationship between polyamine content and dietary pattern using the food supply database of 49 Western countries from the Food and Agriculture Organization of the United Nations.The study was an ecological investigation, and the data used do not indicate the amount of foods actually consumed, however, the food supply must refect the food demand, and thus, we examined the following relationships: the calories of specifc supplied foods relative to the total calories of all supplied foods, and the amount of polyamines contained in specifc supplied foods relative to the total calories in all supplied foods. Mediterranean diet preferred in Mediterranean area was associated with an increased amount of polyamines on a per calorie basis (Binh et al. 2011). Epidemiological analyses and interventional trials have shown that diferences in food preferences and dietary patterns are among the many life-style factors that may play a role in the inhibition of aging-associated diseases and senescence. For example, increased consumption of soybeans and their byproducts is associated with a decreased incidence of cardiovascular diseases (CVDs) (Nagata et al. 2017) and malignancies such as breast (Wu et al. 2008) and colon cancer (Yang et al. 2009). The Mediterranean diet and increased vegetable intake are also associated with a decreased incidence of lifestyle-related diseases, such as CVDs (Estruch et al. 2018) and breast and colon cancer (Couto et al. 2011).
We found that healthy foods, such as germ and bran, legumes such as soybeans, vegetables, and shellfsh, are rich in polyamine on a calorie basis (Soda et al. 2017), and the preference of polyamine-rich diet has a close association with the low incidence of CVDs and with long life span (Soda et al. 2012).
Polyamine intake and polyamine levels in the body
The ability of polyamine synthesis decrease with aging due to the decreased enzymatic activities for polyamine synthesis. However, the age-dependent decreases in polyamine concentrations were not found during adulthood. Instead, there is a large inter-individual diferences in blood polyamine concentrations (Fig. 1). The loss of age-associated decline is considered because polyamine is supplied from intestinal lumen i.e. dietary polyamine and polyamine produced by microbiota and intestinal mucosa. The exact biological mechanisms underlying the large inter-individual diferences in blood polyamine concentrations are not known, however, one factor is thought to be diferences in the amount of polyamines supplied from the intestinal lumen and in the intestinal environment that are also likely to afect polyamine synthesis.In fact, suppression of the polyamine supply from both foods and the intestinal microbiota results in decreased blood polyamine concentrations (Cipolla et al. 2003; Nishimura et al. 2001).Nishibori et al. analyzed polyamine concentrations in Japanese food and estimated that putrescine, spermidine, and spermine account for 45, 37, and 18% of polyamine intake by Japanese, respectively, and the ratio of spermidine and spermine intake was 2:1 (Nishibori et al. 2006).
Bardocz et al. estimated that putrescine (57%) was also the most commonly consumed polyamine in Europeans, with spermidine and spermine accounting for 26 and 18%, respectively; the ratio of spermidine to spermine intake was about 1.5:1 (Bardocz et al. 1995). Spermine and spermidine in the intestinal tract are absorbed quickly, leading to a rapidly increase intestinal vein concentrations (Uda et al. 2003) and distribution to all organs and tissues (Bardocz et al. 1990, 1995). Considering these experimental results, it is logical that increased polyamine intake from foods increases spermidine supply more than spermine, and spermidine concentrations increase in response to increased polyamine intake. However, short-term increases in polyamine intake have thus far not been shown to elevate either spermidine or spermine concentrations (Brodal et al. 1999; Soda et al. 2009a, b). This indicates that blood polyamine concentrations rise for only a very short time, if any, after increased polyamine supply from the intestinal lumen.. Further, rigorous mechanisms of polyamine homeostasis immediately degrade polyamines and maintain their intracellular concentrations. We evaluated the efects of long-term increases in polyamine intake in mice and humans. First, we evaluated the efects of high polyamine chow, which had a polyamine content approximately three to four times higher than regular chow, on blood polyamine levels of mice. The high-polyamine chow was prepared by adding synthetic spermine, spermidine, and putrescine to regular animal chow, and contained spermidine concentrations about four times higher than that of spermine (Soda et al. 2009b).
Increased polyamine intake for 26 weeks increased blood spermidine and spermine concentrations in mice, and the statistical signifcant diference was observed in both spermidine and spermine concentrations (Soda et al. 2009b) or only in spermidine concentrations (Soda et al. 2009a). However, we used only 6–9 animals for the evaluation of blood polyamine concentrations in each group. Later analysis with an increased number (n= 12) of mice fed high polyamine chow for 56 weeks showed that both spermine and spermidine concentrations were increased, as observed in our previous studies, but statistically signifcant increase was observed only in spermine levels (Soda et al. 2013). Since there is wide inter-individual variation in blood polyamine concentrations, it is likely that in the preliminary studies, animals with higher spermidine concentrations after consuming high-polyamine chow probably had higher concentrations before the dietary change (Fig. 1). It is very difcult to observe temporal changes in blood polyamine concentrations in small animals. Therefore, to examine changes in blood polyamine concentrations in response to increased polyamine intake, we performed human interventional trials. Natto is a polyamine-rich food with a ratio spermidine and spermine of about 3:1.Our preliminary study showed increase in spermine levels after increased Natto intake, however spermidine levels did not increase (Soda et al. 2009b). Moreover, we further examined the efect of increased Natto intake on the changes in blood polyamine concentrations (30 male volunteers in intervention group and 27 male volunteers in control group) for 12 months.
For the study, we developed new “Natto” by using polyamine rich soybeans and employing production methods suitable for polyamine-rich natto. All of the used soybeans and fungi were not transgenic. The selected “Natto” for the study contains 390 nmol/g of spermine and 1880 nmol/g of spermidine (Kobayashi et al. 2017). The increased amounts of spermine and spermidine intake estimated by meal records in the intervention group increased (22.00 ± 9.56 and 96.63 ± 47.70 µmol per day, respectively), while no changes were observed in control group. Because the amounts of spermine and spermidine intakes by Japanese were estimated to be 36 and 74 µmol per day (Nishibori et al. 2006), respectively, polyamine intake by volunteers was estimated to be almost doubled during the intervention. Spermine concentrations in whole blood gradually increased following Natto intake and was signifcantly higher than that in the control group by the end of the 12-month intervention, while blood spermidine concentration showed no change (Soda et al. unpublished).These studies clearly showed that increasing the dietary polyamine supply for at least several months gradually increased blood spermine concentrations in humans and mice (Soda et al. 2013; Soda et al. unpublished), despite the fact that foods contain more spermidine than spermine. The mechanism whereby spermine concentrations are increased by elevated polyamine intake is not known.However, extracellular polyamine supply has a signifcant efect on intracellular polyamine concentrations, a phenomenon that is typically seen in cancer patients.
Factors that may afect aging‑associated pathologies
Several food components are considered to inhibit agingassociated pathologies and to help extend life span. Among these substances, antioxidant polyphenols and vitamins are considered to be important candidates for healthy lifespans. The molecules have many biological activities that are likely to inhibit aging-associated pathologies and help extend life span. They have antioxidant and anti-infammatory properties, activate autophagy (Ferraresi et al. 2017; Sacks et al. 2006; Wang et al. 2018b; Zhang et al. 2018), and protect cells and genes from harmful stimuli (George et al. 2017; Guthrie et al. 2017). Early animal experiments and research performed under specific conditions or in particular animals demonstrated that the increased intake of polyphenols extended lifespans. However, many studies have failed to show any efects on the prevention of aging-associated pathologies and the extension of lifespan (Burnett et al. 2011; Sacks et al. 2006; Staats et al. 2018; Strong et al. 2013). In addition, vitamin E and β-carotene, two antioxidant vitamins with potent antioxidant properties, increased rather than decreased the incidence of CVDs and their related mortality (Cook et al. 2007; Miller et al. 2005; Vivekananthan et al. 2003). The study results obtained by numerous investigations indicate that these biological activities are not sufcient to inhibit aging-associated pathological changes or help extend lifespan. Spermine and spermidine have biological activities similar to the abovementioned substances, and therefore it is unlikely that polyamine intake extends lifespan via these activities.
A growing number of recent studies have shown a close relationship between aging and gene methylation (Kochmanski et al. 2018). Gene methylation is one of the mechanism for regulating gene expression without afecting gene sequence. A gene is comprised of combinations of four bases: adenine, guanine, thymine, and cytosine. Gene methylation is a change that involves only cytosine and creates gene information by adding a methyl group from S-adenosylmethionine (SAM) to cytosine residues at the C-5 position to yield 5-methylcytosine. Upstream of the gene, there is a direct repeat of cytosine and guanine called a CpG island. A CpG island is a site of transcription initiation, and in mammals, methylated cytosine within a CpG island can turn the gene of. Conversely, demethylation of cytosine initiates and enhances transcription, resulting in the increased production of the protein encoded by the gene (Fig. 3). Aging is associated with enhanced demethylation of DNA in various organs and tissues in several animals and humans (Avrahami et al. 2015; Nguyen et al. 2016). However, increased hypermethylation associated with age has also been reported in some genes (Khalil et al. 2016; Thalheim et al. 2018).The aging-associated changes in DNA methylation status, namely increased de-methylation in some areas and hyper-methylation in other areas, are considered to be among the most important mechanisms underlying aging-associated pathologies. When hypermethylation arises in the CpG islands encoding genes that suppress agingassociated disease(s) and/or when demethylation arises in the CpG islands encoding genes that cause aging-associated disease(s), the onset and the progression of aging-associated disease(s) are accelerated.
Alteration of methylation status with aging changes chromatin accessibility, resulting in aberrant gene transcription, as well as genomic instability. These factors may be key regulators of the aging process and contributors to the development of aging-associated diseases (Cruickshanks et al. 2013; Lopez-Otin et al. 2013; Watson et al. 2016), including neoplastic growth (Kresovich et al. 2018; Meliso et al. 2017) and aging itself (Ianov et al. 2017; Spiers et al. 2016). Various environmental factors have been reported to afect epigenetic alterations. For example, exposures to fne particulate air pollution, cigarette smoking, and alcohol consumption afect the DNA methylation status (de Lichtenfels et al. 2018; Gao et al. 2017; Liu et al. 2018; Wang et al. 2018a). In particular, cigarette smoking that has adverse efects on health that are associated with changes in epigenetic marks. Smoking-associated changes in methylation status are observed in genes related to the progression of CVDs (Zhang et al. 2016), malignant transformation (Vaz et al. 2017; Zhang et al. 2016), and age acceleration (Gao et al. 2017). Conversely, lifestyles that have favorable efects on health, such as moderate exercise alters epigenetic marks in human skeletal muscle and adipose tissue (Denham et al. 2015; Maejima et al. 2018), and nutritional habits change the methylation status of the promoter area (Barres et al. 2013). And these efect of exercise on improved cardiorespiratory ftness and running performance is accompanied by demethylation of several CpG islands, which is the opposite of the hypermethylation changes observed during aging (Denham et al. 2015; Maejima et al. 2018). However, the mechanism by which life style afects the status of DNA methylation is not known.