Biological Effects of Polyamines on the Prevention of Aging-associated Diseases and on Lifespan Extension
Healthy foods such as beans, mushrooms, vegetables, and seafood and healthy dietary patterns such as the Mediterranean diet and Japanese food have higher concentrations of polyamines (spermine and spermidine). The continuous intake of high-polyamine foods has been shown to increase whole blood polyamine levels in mice and humans. In addition, high-polyamine chow inhibited aging-associated pathological changes in Jc1:ICR male mice and extended their lifespan. Aging is accompanied by decreased DNA methyltransferase activities, increased proinflammatory status, and enhanced abnormal gene methylation status, which is considered to be part of the pathogenesis of aging-associated diseases. In vitro and in vivo experiments have shown that polyamine supplementation reversed such changes induced by aging and polyamine-deficiency. In addition, polyamines have many biological activities that may contribute to the inhibition of lifestyle-related diseases such as diabetes, hyperlipemia, and arteriosclerosis. The possible role of dietary polyamines in human health is discussed.
Introduction
Many epidemiological studies have shown that several foods and dietary patterns have a close association with the inhibition of agingassociated diseases, such as cardiovascular diseases (e.g., myocardial infarction, cerebral infarction) and some types of cancer such as breast and colon cancers. Food preferences and dietary patterns differ widely among countries and regions, emphasizing the role of food components in the inhibition of aging-associated pathologies. The role of antioxidants such as polyphenols (e.g., isoflavone, resveratrol) on human health and longevity has been examined extensively; however, it has not been universally accepted that antioxidants contained in foods help to suppress the occurrence of aging-associated diseases and extend the lifespan (Couzin-Frankel, 2011; Strong et al., 2013). My colleagues and I have demonstrated that polyamines (i.e., spermine and spermidine) are abundant in healthy foods such as beans, vegetables, fish and shellfish and healthy dietary patterns such as Japanese food and the Mediterranean diet (Soda, 2010b, 2011b). We have also shown that in mice, a lifelong consumption of polyamine-rich chow inhibits aging-associated pathological changes in organs and extends the lifespan (Soda, 2009, 2010a, 2012; Soda, Dobashi et al., 2009; Soda et al., 2013; Soda, Kano et al., 2009). Compared to lower organisms such as yeast, nematodes and flies, mammals such as mice and humans have far more advanced and complicated neurological, endocrine and immune functions, and longer lifespans. In industrial countries, the occurrence of many aging-associated diseases shorten the human lifespan, including hypertension, diabetes, hyperlipemia and gout as well as cancers such as colorectal and breast cancers.
The pathogeneses of these diseases are not alike but their progression and severity can significantly affect the human lifespan. It is thus unlikely that a single gene or only a few genes have pivotal roles in the occurrence and progression of all aging-associated diseases (Burnett et al., 2011; Gierman et al., 2014). In this review, I describe polyamine-induced biological activities that may help improve health and extend the lifespan of humans, especially the activities promoted as a result of increases in polyamine concentrations, i.e., via a high-polyamine diet.
Polyamines
Polyamines, i.e., spermine and spermidine, are found in almost all living organisms, and thus foods that are comprised of various types of organisms and their products contain polyamines, which can vary widely in concentration (Cipolla et al., 2007; Nishibori et al., 2006; Nishimura et al., 2006; Soda, 2012). Figure 1 shows the pathway of polyamine metabolism and catabolism as well as polyamine transport. Polyamines are synthesized from cellular arginine. Spermidine has three amino groups (-NH2), while spermine has four. The molecular weight of the largest human polyamine, spermine, is approx. 200 g/mol. The chemical compound putrescine has two amines and is therefore a diamine, and its biological activities differ from those of polyamines. For example, whereas spermine and spermidine have anti-inflammatory activities and are absorbed quickly from the intestinal lumen, putrescine has no anti-inflammatory properties and is degraded predominantly in the intestinal lumen (Soda, 2009; Soda et al., 2005; Zhang et al., 1997; Bardocz et al., 1990; Bardocz et al., 1995).
The enzymatic activities related to polyamine synthesis, especially those of the enzyme ornithine decarboxylase (ODC), decrease with aging (Ferioli et al., 1976). The activities of ODC, which is a rate-limiting enzyme with a short half-life, can be stimulated by specific stimuli (Ferioli et al., 1976; Janne & Raina, 1969; Russell et al., 1970). Because spermidine synthase and spermine synthase lack a regulatory or rate-limiting role in polyamine synthesis, these enzymes have attracted less attention than ODC, and their properties remain to be fully clarified. However, administration of arginine or ornithine stimulated putrescine levels in elderly people and animals, whereas polyamine synthesis was not necessarily stimulated (Bedford et al., 1988; Schleiffer et al., 2000; Teixeira et al., 2002; Yoshinaga et al., 1993). These findings indicate that the activities of spermine and spermidine synthases decrease gradually with aging and without being revitalized. In animal tissue, an aging-associated decline in ODC activity is observed, with a concomitant gradual decrease in polyamine concentration (Das & Kanungo, 1982; Laitinen et al., 1982). However, when polyamine concentrations in whole blood (mainly in erythrocytes and leukocytes) are measured in adult humans, the aging-associated decline in polyamine concentrations is not remarkable, and large inter-individual differences are found (Elworthy & Hitchcock, 1989; Soda et al., 2005).
Cells can synthesize polyamines intracellularly as well as take up polyamines from the extracellular space through a polyamine transporter on the cell membrane. Major sources of body polyamines in adult humans are thought to be those contained in foods and synthesized by the intestinal microbiota. Polyamines in the intestinal tract are absorbed quickly and distributed to almost all organs and tissues of the body (Bardocz et al., 1990; Bardocz et al., 1995). The exact biological background and mechanisms of the large inter-individual differences in blood polyamine concentrations in humans are not known. However, one of the reasons for the variability is thought to be differences in the amount of polyamines supplied by the intestinal lumen, which may reflect individual food preferences as well as the ability of the intestinal microbiota to synthesize polyamines, likely related to the intestinal bacterial flora composition. In fact, when the polyamine supply from foods as well as from the intestinal microbiota is suppressed, polyamine concentrations in whole blood are decreased (Cipolla et al., 2003; Nishimura et al., 2001), and conversely, when an increased polyamine supply from foods is ongoing, blood polyamine concentrations gradually increase (Soda, Dobashi et al., 2009; Soda, Kano et al., 2009). We have shown that upon stimulation with lipopolysaccharide and phorbol 12-myristate 13-acetate, polyamines suppress the production of proinflammatory cytokines from immune cells (Zhang et al., 1997). In addition, polyamines decrease the amount of lymphocyte function-associated antigen 1 (LFA-1) on the surface of immune cells (Soda, 2009; Soda et al., 2005) (Fig. 2a).
LFA-1, the amount of which increases with aging, is one of the phenomena of immuno-senescence, indicating aging-associated changes in immune functions (Chiricolo et al., 1995; Okumura et al., 1993; Pallis et al., 1993; Powers et al., 1992) (Fig. 2b). LFA-1 on immune cells preferentially binds to intercellular adhesion molecules (ICAMs) on endothelial cells lining the blood vessels. This binding activates immune cells and induces the production of various chemical substances including proinflammatory cytokines. Almost all aging-associated diseases are considered to be induced by chronic (repeated and mild) inflammation, as a result of sustained immune cell activation upon stimulation by degraded cells and endogenous pro-inflammatory substances. Therefore, the increased levels of LFA-1 in the elderly indicates the hypersensitivity of immune cells to such originally inoffensive stimuli, and this hypersensitive condition tends to promote the occurrence of and accelerate the progression of aging-associated diseases. Although polyamines suppress the production of proinflammatory cytokines from immune cells upon stimulation and decrease the amount of LFA-1 protein on non-stimulated immune cells, increases in polyamine concentrations enhanced the blastogenic response of immune cells to mitogens such as phytohemagglutinin (PHA) and concanavalin A (Con A) in vitro (Soda et al., 2005). Lymphocyte blast transformation is a method of detecting the potential of immune cell activity. Notably, in the elderly, the blastogenic response of lymphocytes to mitogen is low and the amount of LFA-1 on immune cells is high (Chiricolo et al., 1995; Franceschi et al., 2000; Gillis et al., 1981; Pisciotta et al., 1967; Powers et al., 1992).
In addition, it was shown that polyamine extends the lifespan of cultured immune cells (Eisenberg et al., 2009). We have also found that polyamine supplementation inhibits decreases in the natural killer (NK) activities of immune cells obtained from peripheral blood and cultured (Soda, 2009, 2011a). Polyamines also have anti-oxidant, radical scavenger properties and other biological activities that help protect cells and genes from harmful stimuli (Belle et al., 2004; Brune et al., 1991; Chattopadhyay et al., 2003; Chiu & Oleinick, 1998; Douki et al., 2000; Farbiszewski et al., 1996; Fujisawa & Kadoma, 2005; Gaboriau et al., 2005; Goss et al., 1995; Ha, Sirisoma, et al., 1998; Ha, Yager, et al., 1998; Held & Awad, 1991; Khan et al., 1992; Lovaas & Carlin, 1991; Marzabadi & Llvaas, 1996; Newton et al., 1996, 1997; Rajalakshmi et al., 1978; Sava et al., 2006; Soda et al., 2005; Spotheim-Maurizot et al., 1995; Sy et al., 1999; Tadolini, 1988; Tadolini et al., 1984; Warters et al., 1999; Zhang et al., 1997).
The Relationship between Polyamines and Gene Methylation
A gene is an ‘advanced source of enormous digital information’ comprised of combinations of the four bases adenine, guanine, thymine and cytosine. Gene expression is regulated not only in the ‘digital’ form but also in the ‘analog’ form.
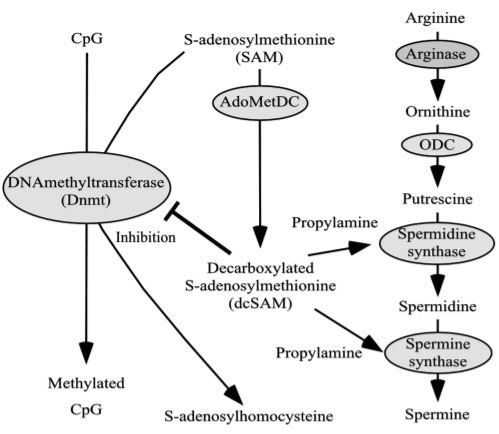
An analog regulatory mechanism is the methylation of genes. Gene methylation is a change that arises only in the base cytosine, creating gene information by adding a methyl group to cytosine. Upstream of the gene information, there is a direct repeat of cytosine and guanine called a CpG island. A CpG island is a site of transcription initiation, and in mammals, methylating cytosine within a CpG island can turn the gene off. Conversely, the demethylation of cytosine initiates and enhances transcription, resulting in the increased production of the protein encoded by the gene (Fig. 3). When methylation arises in the CpG islands encoding genes that function to suppress aging-associated disease(s) and/or when demethylation arises in the CpG islands encoding genes that function to provoke aging-associated disease(s), the onset and the progression of aging-associated disease(s) will be accelerated (Ono et al., 1993; White & Parker, 1983). There is a close relationship between polyamine metabolism and gene methylation (Fig. 3). When spermidine and spermine synthases act to synthesize spermidine and spermine, propylamine is required. Propylamine is supplied from decarboxylated S-adenosylmethionine (dcSAM), which is converted from S-adenosylmethionine (SAM) by the enzymatic activities of S-adenosylmethionine decarboxylase (SAMDC). The methylation of genes indicates the conversion from cytosine to methyl-cytosine by the addition of a methyl group from SAM due to the action of DNA methyltransferase (Dnmt) (Goll & Bestor, 2005). The increase in SAM enriches the supply of methyl groups to a gene,whereas an increase in dcSAM acts to inhibit Dnmt activities (Tsuji et al., 2001; Yamamoto et al., 2010) (Fig. 4).
Enzymatic activities related to polyamine synthesis decrease with aging. To reproduce such a state (of decreased polyamine synthesis) in cultured cells, cells treated with agents that inhibit the activities of ODC or spermine and spermidine synthases or cells deficient in these enzyme activities are used. In cells in which the polyamine concentrations are decreased by overexpressing an antizyme that degrades ODC or by treatment with DL-αdifluoromethylornithine hydrochloride (DFMO), which inhibits ODC activities, or due to a deficit in the activities for spermine synthesis, the intracellular concentrations of dcSAM increase (Frostesjo et al., 1997; Pegg et al., 2011; Shantz et al., 1992; Yamamoto et al., 2010). Simultaneously, such cells have been reported to have enhanced demethylation status of the entire genome (Papazafiri & Osborne, 1987; Tsuji et al., 2001). We found that Dnmt activities were decreased in cells in which the intracellular polyamine concentrations are decreased by DFMO treatment (Kano et al., 2013). In addition, bisulfite sequencing analyses of the LFA-1 gene revealed significant increases in demethylation of promoter regions, especially those responsible for the expression of LFA-1 on immune cells (Richardson, 2002; Zhang et al., 2002) with concomitant increases in the amount of LFA-1 protein (Kano et al., 2013)(Fig. 5). On the other hand, when polyamines are supplied from an extracellular source, the polyamine concentrations are increased, and their increases provoke negative feedback mechanisms that act to inhibit SAMDC activities (Holm et al., 1988; Mamont et al., 1981).
The decreases in SAMDC result in a decreased capability to convert SAM to dcSAM, resulting in increases in SAM and decreases in dcSAM concentrations (Pegg et al., 2011; Yamamoto et al., 2010). polyamine concentrations from extracellular sources seem to activate Dnmt activities (Bestor et al., 1988; Garcea et al., 1989). When 500 µM of spermine was added to cultures of DFMO-treated Jurkat cells, the intracellular spermine and spermidine concentrations and Dnmt activities were increased (Kano et al., 2013). Moreover, in cells supplemented with spermine, the methylation status of the promoter regions of LFA-1 was enhanced and the amounts of LFA-1 proteins were decreased (Kano et al., 2013) (Fig. 5). In an evaluation of the effect of polyamines on LFA-1 expression, we found that the majority of membrane molecules that have physiological roles similar to those of LFA-1 were not influenced. LFA-1 suppression by polyamines was observed to be dose- and time-dependent; the decrease in LFA-1 protein was not observed within 24 h but was apparent after 72 h (Soda et al., 2005). Moreover, Ras-proximate-1 (Rap1), which is an intracellular signal involved in LFA-1 expression, was not affected by polyamines (Kano et al., 2013). These results indicate that the suppression of LFA-1 by polyamines is caused by changes in the methylation status of the promoter region of LFA-1 gene (ITGAL). Although the methylation pattern on the genome, once attached, is generally stably inherited by the next-generation cell (Hashimoto et al., 2010), it has also been reported that the methylation status of some gene regions changes reversibly (Kangaspeska et al., 2008; Kim et al., 2004; Yamamoto et al., 2010). We propose that the promoter region of LFA-1 gene is one such reversible area.
Gene Methylation and Food
Since polyamine is one of the food ingredients absorbed directly from the intestinal tract, it is of great interest that a food ingredient can affect the methylation status of a gene. It was reported that diet or dietary ingredient(s) other than but related to polyamines exerted changes on the methylation status of a gene; for example, deficiency in the supply of methyl groups in foods resulted in the enhanced demethylation of the global genome. Moreover, it was reported that a diet deficient in methyl groups provoked the demethylation of c-myc, c-fox, H-ras, and p-53 genes (Bhave et al., 1988; Christman et al., 1993; Dizik et al., 1991; Pogribny et al., 1995; Zapisek et al., 1992). In other studies, supplementation of methyl groups affected the methylation status and gene expression of several genes (Garcea et al., 1989; Kano et al., 2013).
Aging and Gene Methylation
The demethylation of genes in salmon, mouse, rat, cow, and in human is enhanced with aging (Golbus et al., 1990; Romanov & Vaniushin, 1980; Vanyushin et al., 1973; Vanyushin et al., 1970; Wilson et al., 1987; Zhang et al., 2002). However, aging-associated increases in the methylation of some genes are also reported (Issa et al., 1994; Issa et al., 1996; Wallace et al., 2010). Generally, ODC (Minois et al., 2011) and Dnmt (Lopatina et al., 2002; Oliveira et al., 2012; Romanenko et al., 1998) activities are decreased, and abnormal methylation status (increases in demethylation and methylation) is increased with aging (Kim et al., 2004; Li et al., 2010; Morgan et al., 2005).
Because there is a close relationship between Dnmt activities and the methylation status of LFA-1 promoter regions, and since aging is associated with decreased Dnmt activities as well as increased demethylation of the LFA-1 promoter region (Zhang et al., 2002), the agingassociated enhancement of LFA-1 expression seems to be due to the age-dependent decreases in Dnmt activities. However, as shown in our studies (Kano et al., 2013; Soda et al., 2005), polyamines enhanced Dnmt activities and decreased LFA-1 expression in vitro, suggesting that polyamines counteract agingassociated alterations. In fact, in mice fed high-polyamine chow, the aging-associated increase in LFA-1 (CD11a and CD18) expression -especially increases in the number of bright CD11a cells- was inhibited (Soda et al., 2013). Polyamines activate Dnmt activities, enhances the methylation of the LFA-1 promoter region, and decreases the amount of LFA-1 protein (Kano et al., 2013) (Fig. 5). However, our studies also showed that the activation of Dnmt did not cause the entire promoter region of LFA-1 to exhibit an increased methylation tendency, but it did enhance the demethylation in some parts of the region (Kano et al., 2013) (Fig. 5). We then investigated the influence of polyamines on the methylation status of the whole genome. The restriction enzyme Not I cleaves Not I sites located throughout the genome, but when cytosine in the Not I site is methylated, Not I fails to cleave it. Depending on the region, the decrease in Dnmt activities induced by a reduction in polyamines, not only provoked increases in the demethylation of some parts of the genome but also reinforced the methylation in other regions.
Namely, polyamine deficiency provoked both increased demethylation and increased methylation, resulting in an abnormal methylation status of the whole genome (Soda et al., 2013) (Fig. 6). The abnormal methylation status was reversed by an increase in polyamine concentration, by the addition of spermine via an extracellular route. Although the Not I site is not necessarily involved in the gene expression, the methylation status of 10% of the gene fragment cleaved at the Not I site was influenced by the changes in polyamine concentration (Soda et al., 2013). That is, in about 5% of the fragment, polyamine supplementation reversed the increase in demethylation induced by the decrease in polyamine concentration, while in about 5% of the fragment, polyamine supplementation reversed the increase in methylation induced by the decrease in polyamine concentration. Because abnormal gene methylation is associated with agingassociated diseases and aging (Borghini et al., 2013; Maegawa et al., 2014; Ono et al., 1993; Ushijima & Okochi-Takada, 2005; White & Parker, 1983), an elevation in polyamine concentrations by replenishment from foods must regulate the methylation status of various genes relevant to the onset or the inhibition of aging-associated diseases. In addition to the many biological activities that help inhibit damage to cells and genes caused by harmful stimuli, such biological effects on gene methylation have contributed to the lifespan extension of mice (Soda, Dobashi et al., 2009; Soda et al., 2013).