Surprise for a long life
Identifying therapies to slow down ageing and delay age-associated diseases is a primary goal of ageing-related research. Resveratrol and rapamycin were first found to promote longevity in yeast, and their effects were then extended to several organisms. Spermidine is a new longevity drug that can increase life span in yeast, nematodes and flies, possibly through an effect on chromatin-mediated regulation of gene expression.
Spermidine is a naturally occurring polyamine essential for life1 . Two additional polyamines, putrescine and spermine, act with spermidine to modulate diverse cellular processes, including DNA stability, transcription, translation and apoptosis. On page 1305 of this issue, Eisenberg et al. establish an important new role for spermidine in ageing2 . They show that supplementation with spermidine increases life span in yeast, nematodes and flies and improves survival of human immune cells in culture. A model for how spermidine slows ageing is proposed, involving global remodelling of chromatin leading to enhanced degradation of damaged macromolecules and increased resistance to oxidative stress. Previous studies had indicated that polyamines decline with age in humans, but it was unclear whether this decline directly contributed to ageing2 . To test this possibility, Eisenberg et al.2 turned to the yeast chronological ageing model, in which cells are aged in a non-dividing, post-mitotic state3. They observed that endogenous spermidine declines in chronologically ageing yeast, but that adding it to the growth medium could reverse this decline and extend life span2. Yeast chronological ageing is caused by accumulation of acetic acid in the medium, which induces an apoptotic-like response3 . Spermidine can protect mammalian cells from apoptosis1 , suggesting a possible mechanism of life-span extension. Consistent with this idea, endogenous spermidine was required for protection against acetic acid-induced apoptosis in ageing cells; however, addition of spermidine also increased chronological life span when acetic acid was removed from the ageing culture, indicating that additional mechanisms beyond suppression of apoptosis are likely to be involved2 .
What might these additional mechanisms be? Eisenberg et al. propose that spermidine promotes longevity by upregulating the lysosomal/vacuolar degradation pathway, referred to as autophagy, and that this leads to enhanced resistance to oxidative stress and decreased cell death. This is an attractive model, as induction of autophagy has been implicated in life-span extension in yeast, nematodes and flies4,5. Importantly, in addition to increasing chronological life span in yeast, spermidine also increased life span in both nematodes and flies, and the life span extension in all three organisms was impaired by inhibition of autophagy. Eisenberg et al. propose that spermidine induces autophagy by inhibiting histone acetyltransferases. Generally, hyperacetylation of histones is associated with higher levels of gene expression, whereas hypoacetylation of histones is associated with gene silencing. Eisenberg et al. show that spermidine can inhibit acetyltransferases in vitro, and that treatment of cells with spermidine leads to a global decrease in histone H3 acetylation. Paradoxically, they found that promoters of at least some autophagy-related genes are hyperacetylated by spermidine, which results in increased transcription and activation of the autophagic machinery. The mechanistic basis for differential modification of chromatin near autophagy-related genes remains unclear, but may involve a regulated response to global hypoacetylation.
A particularly intriguing possibility that was not explored in this study is that spermidine is acting as a dietary restriction mimetic. Dietary restriction has been shown to slow ageing in many different organisms from yeast to primates6,7, and the anti-ageing effects of dietary restriction seem to be mediated, at least in part, by inhibition of TOR (target of rapamycin) kinase8 . In addition to regulating mRNA translation, a primary function of TOR signalling is to repress autophagy5 , and (like spermidine) blocking autophagy reduces the life-span extension associated with either dietary restriction or TOR inhibition5,8. This raises the question as to whether spermidine can directly inhibit TOR. One argument against such a TORdependent mechanism is that spermidine failed to increase the replicative life span of yeast cells when supplementation was initiated early in life2 . Both dietary restriction and TOR inhibition increase replicative life span (defined as the number of daughter cells produced by a mother cell before senescence) by a mechanism likely to involve a reduction in mRNA translation8 . Spermidine did, however, partially restore the replicative potential of aged yeast cells when supplementation was started late in life2 , suggesting that there may be a complex interaction between TOR signalling, autophagy and spermidine.
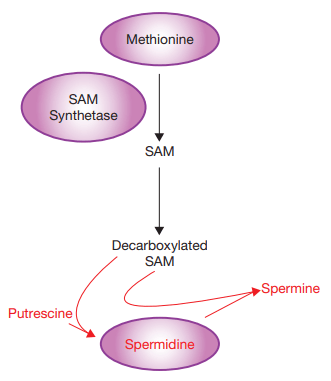
Other than upregulation of autophagy, additional processes are also likely to contribute to spermidine effects on longevity. For example, spermidine can interact with from S-adenosylmethionine1 , and decreased expression of S-adenosylmethionine synthetase is sufficient to increase life span in both yeast and nematodes9 , while methionine restriction slows ageing in rodents10. As S-adenosylmethionine is the primary methyl donor involved in histone methylation, another covalent modification of chromatin important for regulating gene expression, it would be of interest to determine whether global changes in histone methylation accompany the hypoacetylation of histones associated with spermidine supplementation.
A key question for the future is whether the effects of spermidine on longevity are conserved in mammals. It was recently shown that dietary supplementation with the TOR inhibitor rapamycin increases life span in mice, even when initiated in middle-age11. It would be particularly interesting to know whether spermidine has a similar effect. Eisenberg et al.report that replicative capacity of human immune cells in culture is increased by spermidine, but this is not a convincing indication that ageing will be delayed in vivo across a variety of tissues. Interestingly, mice fed a high polyamine diet show indications of increased longevity and reduced age-associated pathology in some tissues, particularly kidney12. The longevity data for polyamine-supplemented mice is difficult to interpret with confidence, however, as the experiment was not complete at the time of publication and the control animals have a remarkably short median life span of less than 80 weeks12. It will be important for future studies to more carefully examine the effects of spermidine on mouse life span in a longer-lived strain background. It will also be informative to understand how spermidine influences health span in mammals.
What is the potential relevance of this study for human ageing? Clearly, if spermidine is found to increase life span or health span in rodents, it will join rapamycin and resveratrol as a leading candidate for treating age-associated diseases in people. Of these, rapamycin is currently the only one known to enhance mouse longevity, but there are concerns that rapamycin may be of limited therapeutic value due to detrimental side effects such as immunosuppression13. No such side effects are known for spermidine. In addition, spermidine is a natural component of our diet, and several foods are known to be rich in spermidine, including soy beans, tea leaf, and mushrooms. Evidence suggests that eating a diet rich in spermidine results in increased blood spermidine levels14. Thus, it could be relatively easy for most people to obtain the benefits of spermidine through dietary modifications or by supplementation. However, there may be a darker side to spermidine with respect to mammalian ageing: oxidation of polyamines causes oxidative stress that can induce cell death, and high levels of polyamines are associated with malignancy15. Indeed, chemical inhibitors of polyamine metabolism are being studied as potential anti-cancer agents15. Thus a better understanding of the mechanistic details that underlie the pro-longevity effects of spermidine in simple eukaryotes, as well as careful long-term studies of dietary supplementation with spermidine in mammals, are needed before such a strategy should be pursued in humans.