A novel autophagy inducer and longevity elixir
Spermidine is a ubiquitous polycation that is synthesized from putrescine and serves as a precursor of spermine. Putrescine, spermidine and spermine all are polyamines that participate in multiple known and unknown biological processes. Exogenous supply of spermidine prolongs the life span of several model organisms including yeast (Saccharomyces cerevisiae), nematodes (Caenorhabditis elegans) and flies (Drosophila melanogaster) and significantly reduces agerelated oxidative protein damage in mice, indicating that this agent may act as a universal anti-aging drug. Spermidine induces autophagy in cultured yeast and mammalian cells, as well as in nematodes and flies. Genetic inactivation of genes essential for autophagy abolishes the life span-prolonging effect of spermidine in yeast, nematodes and flies. These findings complement expanding evidence that autophagy mediates cytoprotection against a variety of noxious agents and can confer longevity when induced at the whole-organism level. We hypothesize that increased autophagic turnover of cytoplasmic organelles or long-lived proteins is involved in most if not all life span-prolonging therapies.
A decrease in cellular polyamine content has been repeatedly correlated with aging. Therefore, we tested the effects of external spermidine administration on aging, using yeast as a model. When applied at millimolar concentrations, spermidine efficiently increased chronological life span (that is the time that a yeast culture remains viable in stationary phase) while it retarded necrotic cell death. In addition, spermidine could rejuvenate replicatively old yeast cells as their replicative life span was increased (that is the number of daughter cells generated from one single mother cell). Chronological aging is conceived as a model of aging affecting postmitotic cells from animals, whereas replicative aging is thought to reflect the aging process of proliferating cells such as stem cells. Driven by the discovery that spermidine improved both the chronological and the replicative life span of yeast cells, we tested its effect on the aging of several animal species. We found indeed that spermidine had a beneficial antiaging effect on nematodes and fruit flies when added to their food, again at millimolar concentrations. Moreover, addition of spermidine could retard the spontaneous loss of viability of human peripheral blood mononuclear cells (PBMC) in vitro. This effect was obtained at relatively low concentrations (around 20 nM), and it is not entirely clear whether this reflects a similar mode of action as that obtained in whole organisms. When mice were supplied with 3 mM spermidine in the drinking water, they manifested reduced age-associated protein oxidation, which is one of the hallmarks of aging. Altogether, these results illustrate spermidine’s potential to promote longevity.
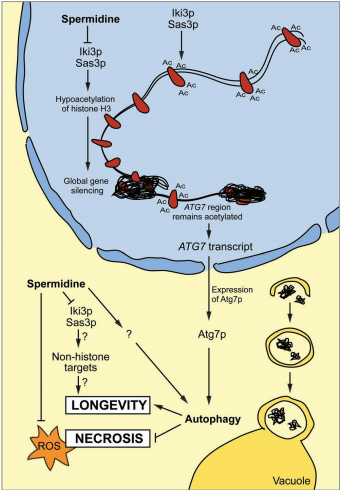
Encouraged by the aforementioned observations, we decided to determine the mechanisms through which spermidine might exert its anti-aging effect. Driven by the knowledge that sirtuins may promote longevity by catalyzing the deacetylation of histones, we investigated whether spermidine may affect the acetylation of histones as well. We observed that spermidine treated yeast cells exhibited a marked hypoacetylation of histone H3 at all acetylation sites located at the amino terminal tail (Lys 9, 14 and 18). Given that spermidine exerted its life-span extension also in yeast cells that lack the ortholog of sirtuin 1 (SIR2), we postulated that spermidine must affect another enzyme that catalyzes histone (de)acetylation.
Accordingly, we found that spermidine directly inhibited the enzymatic activity of histone acetyl transferases (HAT) in an in vitro HAT activity assay. Importantly, spermidine also reduced the acetylation of histone H3 in human PBMC cultured in its presence and changed the acetylation pattern of tissues from mice that received oral spermidine. This suggests that histone H3 deacetylation is a general correlate of the life spanextending action of spermidine. In yeast cells, spermidine causes hypoacetylation of histone H3 associated with several promotor regions (adjacent to ATG7), while the promotor region of ATG7 itself . remained selectively acetylated, allowing for its transcription in a state of general gene silencing. The spermidine-driven transactivation of autophagy-related genes indeed induced signs of macroautophagy. In this context it should be noted that Sirtuin-1-induced autophagy has been correlated with the deacetylation of essential autophagy-related proteins such as Atg5 and Atg7. Therefore, it remains to be investigated whether spermidine might induce autophagy by a combination of transcriptional and cytoplasmic (transcription-independent) mechanisms .
Spermidine induced signs of macroautophagy in all systems that we investigated including yeast cells, C. elegans, Drosophila and human tumor cells. Thus, the microtubule-associated light chain 3 (LC3), also called Atg8 protein, redistributed from a diffuse location to a punctate cytoplasmic location, at the surface of autophagosomes. Moreover, LC3/Atg8 underwent lipidation (which increases its electrophoretic mobility), a post-translational modification that is required for its association with autophagosome membranes. Ultrastructural analyses confirmed the formation of characteristic two-membraned vesicles indicative of autophagy in spermidine-treated cells. Since autophagy is impaired in innumerous pathological conditions, medical application of spermidine as a natural autophagy-inducing treatment is of most interest for future research. Hence, its applicability to other cell types and conditions—associated with a variety of pathological conditions that differ from aging—should be tested.
Finally, we found that inhibition of autophagy by knockout of essential autophagy-related genes (such as ATG5, ATG6 (bec-1 in C. elegans) or ATG7) abrogated the life span-prolonging effect of spermidine in yeast, nematodes and flies, indicating that induction of autophagy is indeed essential for the anti-aging activity of spermidine. In this respect, it should be mentioned that pharmacological induction of autophagy using rapamycin also has life span-prolonging effects in yeast, flies and in mice. Moreover, caloric restriction can only prolong the life span of C. elegans that harbor an intact autophagic machinery. These results suggest that autophagy might be a common pathway of several (if not all) life span-prolonging measures. This hypothesis requires urgent exploration.