Induction of autophagy by spermidine promotes longevity
Ageing results from complex genetically and epigenetically programmed processes that are elicited in part by noxious or stressful events that cause programmed cell death. Here, we report that administration of spermidine, a natural polyamine whose intracellular concentration declines during human ageing, markedly extended the lifespan of yeast, flies and worms, and human immune cells. In addition, spermidine administration potently inhibited oxidative stress in ageing mice. In ageing yeast, spermidine treatment triggered epigenetic deacetylation of histone H3 through inhibition of histone acetyltransferases (HAT), suppressing oxidative stress and necrosis. Conversely, depletion of endogenous polyamines led to hyperacetylation, generation of reactive oxygen species, early necrotic death and decreased lifespan. The altered acetylation status of the chromatin led to significant upregulation of various autophagy-related transcripts, triggering autophagy in yeast, flies, worms and human cells. Finally, we found that enhanced autophagy is crucial for polyamine-induced suppression of necrosis and enhanced longevity
As an organism ages, the fate of individual cells is dictated by apoptotic or necrotic cell death pathways, as well as autophagy, as a cytoprotective process1–3. Until recently, necrosis has been regarded as a form of accidental, unregulated cell death resulting from severe chemical or physical disruption of the plasma membrane, which contrasts with the subtly regulated, ‘programmed’ apoptotic death. Recent research suggests, however, that the occurrence and course of necrosis can be subject to complex controlled processes and that necrosis can therefore also be ‘programmed’4,5. During replicative ageing of yeast, activation of the phylogenetically conserved ageing regulator Sir2, an NAD+-dependent histone deacetylase, has been found to promote longevity6 and to be important (among other sirtuins) for lifespan extension under various conditions7,8. Epigenetic hypoacetylation of histones has since been regarded as a key process during healthy ageing6,9–12. Chronological ageing of yeast cells follows molecular pathways that are shared with those dictating longevity of non-dividing post-diauxic cells of higher eukaryotes2,13. One of these pathways is regulated by Tor kinases, and decreased TORC1 activity can promote longevity of various organisms14–16. TORC1 activity is known to negatively regulate autophagy, the major lysosomal degradation pathway that recycles damaged and potentially harmful cellular material. Accordingly, autophagy counteracts cell death and prolongs lifespan in various models of ageing17,18. Among the multiple biochemical correlates of ageing, a decrease in intracellular polyamines has been described in ageing mammalian cell culture and during human ageing in various organs including serum19.
However, it has remained unclear whether depletion of polyamines is a cause or a consequence of the ageing process. Here, we show that administration of exogenous spermidine, a naturally occurring polyamine, extends lifespan in various models of ageing through epigenetic modifications, induction of autophagy and suppression of necrosis.
Spermidine application suppresses ageing in yeast, flies, worms, human cells and mice.
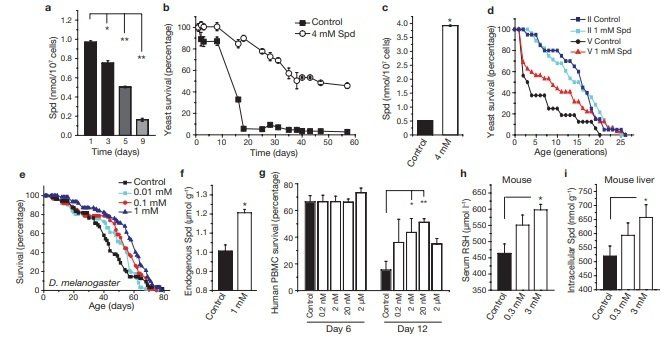
A decrease in polyamines has been repeatedly correlated with the ageing process, although its potential causality has not yet been investigated.To address this, we applied spermidine to chronologically ageing yeast cells, which (as we show here) show a decline in the levels of endogenous polyamines (Fig. 1a) and a progressive loss in clonogenic survival1,2,20. Exogenous supply of spermidine to ageing wild-type BY4741 cells (at day 1) caused a marked increase in lifespan by a factor of up to four times that of untreated cells, as determined in clonogenic assays that monitored the frequency of viable cells (Fig. 1b). Similar results were obtained using wild-type DBY746 cells (Supplementary Information, Fig. S1a). Spermidine supplementation also led to a stable increase in intracellular spermidine levels in ageing cells (Fig. 1c), which would otherwise show a decrease in endogenous spermidine levels (Fig. 1a).
Whereas chronological ageing is a model for ageing of post-mitotic tissues, replicative ageing of yeast models the lifespan of dividing cells in higher eukaryotes. Both ageing systems are interrelated, as replicatively old cells die early during chronological ageing21. We therefore analysed the differential effect of spermidine on replicatively young and old cells obtained by elutriation22. The remaining replicative lifespan of old cells was significantly increased by spermidine (Fig. 1d, fraction V cells, P < 0.02), whereas no apparent effect was seen on the remaining lifespan of replicatively young cells (fraction II cells, Fig. 1d). Thus, spermidine retards chronological ageing and also and without (control) supplementation of food with various concentrations of spermidine (as indicated). A representative ageing experiment of at least 50 flies per sample is shown. (f) Endogenous spermidine of female Drosophila fed for 48 h with food supplemented by 1 mM spermidine, compared with normal food (control). Data represent means ± s.e.m. (n = 3; *P < 0.01). (g) Survival determined by annexin V/7‑AAD co-staining (unstained cells were considered as viable) of human immune cells (PBMC) cultured for 6 and 12 days in the absence (black bar) or presence (white bars) of various spermidine concentrations (as indicated). Data represent means ± s.e.m. of 3 independent experiments (each performed on PBMC from different donors). *P < 0.05 and **P < 0.01. (h, i) Free thiol group (RSH) concentration in serum (h) and intracellular spermidine concentration in hepatocytes (i) of ageing mice with (open bars) or without (closed bar) supplementation of drinking water with spermidine (0.3 and 3 mM) for 200 days. Data represent mean ± s.e.m. (n = 3; *P < 0.05 and **P < 0.01).
rejuvenates replicatively old cells. Improved longevity often correlates with increased stress resistance23. Accordingly, long-lived spermidinetreated cells showed a strong resistance to stress inflicted by heat shock or H2 O2 treatment (Supplementary Information, Fig. S1b). In an attempt to extend the lifespan of a complete metazoan organism, we supplemented ordinary food of the fruitfly Drosophila mela‑ nogaster with spermidine. Optimal doses of spermidine increased the mean lifespan of flies up to 30% (Fig. 1e, P = 0.0002 for 1 mM; for mean lifespans and replicates see Supplementary Information, Fig. S2). Measurement of endogenous polyamines confirmed that spermidine supplementation stably increased intracellular spermidine levels by about 20%, compared with controls (Fig. 1f). Notably, putrescine (a polyamine interconvertable with spermidine) was undetectable in control samples but clearly present in spermidine-fed flies (~100 nmol g–1), indicating that spermidine was indeed taken up and metabolized by the flies (data not shown). Similarly, we found that polyamines prolonged the mean and the maximum lifespan of the nematode Caenorhabditis elegans. Supplementation of regular food with spermidine (0.2 mM) extended the nematode lifespan by up to 15% (Fig. 7i, P <0.0001). We next investigated whether polyamines also enhanced the lifespan of human peripheral blood mononuclear cells (PBMC), and monitored survival using annexin V/7-AAD co-staining (unstained cells were regarded as viable). After 12 days, only 15% of cells in the control PBMC cultures survived, whereas up to 50% of the cells survived after addition of spermidine (20 nM; Fig. 1g).
Unexpectedly, the rescuing effect did not involve any inhibition of apoptosis, as the percentage of apoptotic cells (annexin V+/7-AAD– ) was not influenced by spermidine. Instead, cell death associated with membrane rupture, which is indicative of necrosis (annexin V+/ 7-AAD+ cells), was markedly reduced (Supplementary Information, Fig. S3a).One of the most widely accepted theories of ageing is the free radical theory, which attributes ageing to accumulating oxidative stress24. In rodents, the level of oxidative stress and protein damage increases consistently with age, as observed by a decline in free thiol groups in serum proteins25. Feeding mice with spermidine (3 mM, added to the drinking water) for 200 days increased the serum level of free thiol groups by about 30%, indicating reduced age-related oxidative stress (Fig. 1h). Notably, such an increase in free thiol groups is comparable to the natural decline that has been observed during the course of ageing (between young and old rodents)25. Again, intracellular spermidine levels were significantly increased by exogenous spermidine supplementation, as determined in liver cells (Fig. 1i). Together, these data indicate that exogenous supplementation of spermidine can retard cellular and organismal ageing in several species.
Polyamine depletion decreases yeast lifespan and increases necrosis
We next investigated the effect of polyamine depletion on yeast chronological ageing, using a yeast strain that is deficient in SPE1 (Δspe1) and hence unable to synthesize polyamines. Polyamine depletion, as confirmed by measurement of intracellular spermidine (Fig. 2a), markedly shortened lifespan, which could subsequently be restored by supplementation with 0.1 mM spermidine or its precursor putrescine (Fig. 2b, data not shown). Note that in this case, a low spermidine concentration was chosen for complementation, which did not enhance wild-type survival per se (Fig. 2b). Consistent with the free radical theory of ageing24, we observed an enhanced accumulation of oxygen radicals after disruption of SPE1, as indicated by the increased superoxide-mediated conversion of cell-permeable non-fluorescent dihydroethidium (DHE) to fluorescent ethidium (Eth), which remains trapped in the cells (Fig. 2c, d). Close inspection of phenotypical cell death markers revealed that the enhanced death rate of Δspe1 cells was associated with a rapid loss of membrane integrity, although apoptotic markers remained constant (Fig. 2e; see Supplementary Information, Results and Discussion for more details). We conclude therefore that depletion of intracellular polyamines can precipitate premature chronological ageing through non-apoptotic, presumably necrotic death of yeast cells.
Spermidine prolongs lifespan in various ageing models in a pHindependent fashion
Very few studies have addressed the mechanisms of necrotic cell death in a systematic fashion. In C. elegans, acidification of the cytosol is reportedly required for necrotic cell death, whereas alkalinization has a cytoprotective effect26,27. Furthermore, recent reports indicate that one of the major causes of yeast chronological ageing is the excessive production of acetic acid28. Consistent with this view, administration of spermidine to chronologically ageing yeast, or alkalinization of the medium with NaOH, prolonged yeast lifespan and increased the extracellular and cytosolic pH (data not shown) but in a manner strictly dependent on intracellular polyamines (Supplementary Information, Fig. S3b; see Supplementary Results and Discussion for further details). Chronological ageing of yeast in water, which is independent of the pH because of repeated removal of produced acid28, reportedly relies on phylogenetically conserved molecular mechanisms29 similar to ageing under standard conditions30. We therefore transferred stationary yeast cells preloaded with high concentrations of spermidine (8 mM) to water at an early stage during ageing, while adjusting the pH of spermidine cultures to that of spermidine-free controls. Under these conditions, spermidine continued to extend the chronological lifespan and reduce ROS levels (Fig. 6e, f). In summary, spermidine is able to extend lifespan and to inhibit age-related oxidative stress in a pH-independent fashion.
Spermidine application counteracts age-induced necrotic cell death
Determination of cell death markers revealed that markers of necrosis (loss of membrane integrity) and oxidative stress (DHEEth conversion) were markedly diminished with spermidine treatment (Fig. 3a–c; see Supplementary Discussion for details). Consistently, electron microscopy of old cells (day 20) showed necrotic disintegration of subcellular structures and rupture of the plasma membrane, whereas the ultrastructure of spermidine-treated samples of the same age resembled that of young cells (Fig. 3d). Nuclear release of the high mobility group Box 1 protein (HMGB1), a chromatin bound non-histone protein, is a defining feature of necrosis in mammalian cells31. Fluorescence microscopy detected the nucleo–cytosolic translocation of the yeast HMGB1 homologue Nhp6Ap (rendered visible with an EGFP tag) after 14 days of ageing (Fig. 3e). Again, this necrotic feature was prevented by application of spermidine (Fig. 3e). Thus, spermidine treatment protects against ageing through the inhibition of necrosis.
Hypoacetylation of histone H3 correlates with spermidineinduced longevity
As the budding index and mutation frequency during ageing32 remained largely unaffected by application of spermidine (Supplementary Information, Fig. S3d, e and Results and Discussion), we considered the possibility that epigenetic modifications, rather than regrowth of death-resistant mutants1 , might regulate lifespan extension. Histone deacetylation, a key event in epigenetic chromatin modification9 , is associated with healthy ageing in many organisms11 and deacetylation of specific lysyl residues was suggested to be crucial for yeast lifespan extension, at least during replicative ageing6,12. We therefore analysed the effects of spermidine on the level of histone acetylation by using antibodies that specifically detect acetylated lysyl residues located at the amino-terminal tail of histone H3 (Lys 9, 14 and 18). The improved lifespan of ageing wild-type cells treated with spermidine correlated with hypoacetylation of histone H3 at all acetylation sites monitored (Fig. 4a, b; see Methods and Supplementary Information, Fig. S4 for details of quantification). Conversely, premature death of ageing SPE1-deleted cells was accompanied by hyperacetylation of histone H3.
These results suggest that global deacetylation and polyamines are both connected to the extension of chronological lifespan in yeast, yet cannot serve as a final proof of causality between histone deacetylation and longevity. Interestingly, the level of histone acetylation decreased during ageing of wild-type cells kept under standard culture conditions (Fig. 4d), perhaps as an adaptive anti-ageing mechanism. Accordingly, acceleration of this adaptive response (histone hypoacetylation) by administration of exogenous spermidine led to longevity.
Next, we investigated whether spermidine would affect histone H3 acetylation during ageing in mammalian cells. Exogenous supply of spermidine (20 nM) to human PBMC greatly reduced the acetylation levels of Lys 14 and 18 after as few as 6 days of incubation (Fig. 4e; Supplementary Information, Fig. S4d). Hepatocytes from mice fed with spermidine for 200 days showed similar levels of histone H3 acetylation at Lys 14 or 18, compared with liver cells from control mice (Fig. 4f). However, an electrophoretically more mobile form of histone H3 appeared in extracts from control mice, histone H3 Lys residues of Δspe1 cells (open bars), compared with wildtype cells (closed bars) during chronological ageing. Data represent means ± s.e.m. (n = 3). P values indicate the result of a two-factor ANOVA corrected by Bonferroni post hoc test. (d) Relative acetylation (normalized to day 1) of histone H3 Lys 18 residue of chronologically ageing wild-type cells.
Data represent means ± s.e.m. (n = 3). (e) Relative acetylation (normalized to controls) of histone H3 Lys 14 and 18 residues of cultured human PBMC after 6 days of incubation with or without 20 nM spermidine. Data represent quantification of two independent experiments performed with cells obtained from two different donors. (f) Immunoblot analysis of liver cell extracts (applied in serial dilutions) obtained from mice fed with 3 mM spermidine for 200 days and respective control mice of the same age. Blots were probed with antibodies recognizing total histone H3 (C-terminal epitope) or specific for acetylated lysine 18 residue (N-terminal epitope). Full scans of blots are available in Supplementary Information, Fig. S5.suggesting its proteolytic processing33. Note that we detected the processed form of histone H3 only when using acetylated Lys-specific antibodies that recognize H3 at an N-terminal epitope and not when using the antibody against total histone H3 that recognizes a carboxy-terminal epitope. These observations indicate a C-terminal cleavage of the histone, however, at a different site compared with recent findings in mouse embryonic stem cells33. This physiological age-related processing of histone H3 was completely inhibited by feeding mice spermidine (Fig. 4f). Our findings suggest that spermidine-inducible modification of histone H3, as with yeast lifespan regulation, also coincides with organismal ageing, although in a more complex fashion.
Spermidine inhibits HAT activity, which causes longevity and suppression of necrosis
As the role of the Sir2p deacetylase is well established in replicative ageing7,11,12, we tested its potential involvement (and that of 27 other proteins implicated in histone acetylation) in polyamine-promoted longevity during chronological ageing. Deletion of SIR2 or any other sirtuin did not abrogate the ability of spermidine to extend chronological lifespan (Supplementary Information, Table S1 and data not shown). Instead, the pro-survival effect of spermidine was partially abrogated in two of the screened knockout strains, Δiki3 and Δsas3 (see Supplementary Information, Table S1, Results and Discussion for details). Deletion of IKI3, an essential subunit of the histone acetylating elongator complex, is known to reduce histone H3 acetylation at Lys 14 (ref. 34). Similarly, the HAT Sas3p is known to preferentially acetylate histone H3 at Lys 14 GO terms of macroautophagy (GO:0016236), microautophagy (GO:0016237) or autophagy in general (GO:0006914) at day 3 (e) and day 10 (f) of chronological ageing yeast. Data represent means of two independent arrays from independent biological samples. (g) Relative change of ATG7, ATG11 and ATG15 mRNA levels by spermidine supplementation (normalised to controls) after ten days of chronological ageing as determined by reverse transcriptase real-time PCR. Data represent means ± s.e.m. (n = 3; *P < 0.05 and **P < 0.01). (h) Relative histone H3 Lys 18 acetylation of indicated promoter regions normalized to the acetylation of ATG7 promoter (pATG7) determined by chromatin immunoprecipitation with H3‑K18Ac specific antibody and subsequent quantification of precipitated promoter DNA using quantitative real time PCR.