Spermidine in health and disease
Interventions that delay aging and protect from age-associated disease are slowly approaching clinical implementation. Such interventions include caloric restriction mimetics, which are defined as agents that mimic the beneficial effects of dietary restriction while limiting its detrimental effects. One such agent, the natural polyamine spermidine, has prominent cardioprotective and neuroprotective effects and stimulates anticancer immunosurveillance in rodent models. Moreover, dietary polyamine uptake correlates with reduced cardiovascular and cancer-related mortality in human epidemiological studies. Spermidine preserves mitochondrial function, exhibits anti-inflammatory properties, and prevents stem cell senescence. Mechanistically, it shares the molecular pathways engaged by other caloric restriction mimetics: It induces protein deacetylation and depends on functional autophagy. Because spermidine is already present in daily human nutrition, clinical trials aiming at increasing the uptake of this polyamine appear feasible.
As the world population ages, chronic maladies such as diabetes, cardiovascular disease, cancer, and neurodegeneration become ever more prevalent because life expectancy is increasing at a quicker pace than does health span (1). Interventions that favor healthy aging could constitute powerful strategies to limit human diseases that have a broad socioeconomic impact. Caloric restriction (CR), which is defined as the chronic reduction of calorie intake without malnutrition, is among the few regimens that extend life and beneficially affect health in all tested model organisms, including rodents and nonhuman primates (2, 3). However, it is difficult to set the optimal intensity of CR to avoid undernourishment and other unwanted effects, for which reasons CR is contraindicated in elderly and diseased persons (1, 4, 5). Moreover, only few people seem capable of changing their dietary routines for extended periods (6). Thus, the supplementation of caloric restriction mimetics (CRMs), which would pharmacologically mimic the beneficial effects of caloric or dietary restriction, has gained attention as an attractive and potentially feasible strategy (7). Conceptually, healthy aging requires the attenuation or retardation of several molecular and cellular alterations that drive the aging process
and induce age-associated pathologies. These include genomic instability, telomere attrition, epigenetic alterations, loss of proteostasis, deregulated nutrient sensing, mitochondrial dysfunction, cellular senescence, stem cell exhaustion, chronic inflammation, and altered intercellular communication (2, 8–10). Accordingly, regimens that extend life span of model organisms often display pleiotropic effects that include but are not limited to anti-inflammatory properties, enhancement of mitochondrial metabolic function and respiration, as well as improved proteostasis. This holds true also for the application of CRMs, most of which enhance cytoprotective autophagy (examples of CRMs are summarized in table S1), although additional effects such as on energy metabolism cannot be excluded (11). Autophagy ensures general cell homeostasis and proteostasis and is directly involved in the degradation of damaged, potentially toxic organelles or longlived protein aggregates (12). Through controlled sequestration of cytoplasmic material into doublemembraned autophagosomes, autophagy directs macromolecules (including proteins, lipids, and nucleic acids) or whole organelles to lysosomal degradation. This process detoxifies and recycles potentially harmful material that accumulates during aging. Consistent with its protective function against aging and disease (13–15), autophagy is also required for the life-span–extending effects of CR and of several CRMs (2, 12).
These CRMs mostly induce autophagy via a common mechanism that involves the deacetylation of cytosolic as well as nuclear proteins (7, 16–19). However, the molecular targets accounting for these effects vary among CRMs. CRMs can act as inhibitors of the synthesis of acetyl coenzyme A (CoA), which is essential for enzymatically catalyzed acetylation reactions, for direct inhibitors of acetyltransferases, or as activators of deacetylases (in particular sirtuins) (table S1).The diamine putrescine and the polyamines spermidine and spermine are ubiquitously occurring polycations that are associated with several important cellular functions and help maintain general cell homeostasis. Polyamines are essential for cell growth and proliferation and tissue regeneration. They bind and stabilize DNA and RNA, have antioxidative activities, modulate enzyme functions, and are required for the regulation of translation. These characteristics have been reviewed elsewhere (20–22).
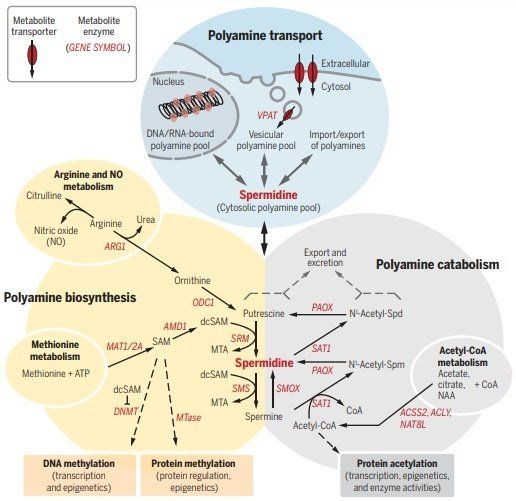
Polyamines have also been characterized in the control of apoptosis (20, 22) and in the context of learning and memory (23). Spermidine acts as a natural autophagy-inducer (24) and antiaging compound that shares many beneficial traits with CR and thus can be considered as a CRM. We review the potential health-promoting effects of polyamines on aging and its comorbidities. Such effects include cardiovascular, neuroprotective, and anti-tumorigenic effects and are induced by dietary or otherwise externally applied spermidine, which will be the primary focus of this article. Spermidine concentrations decline with age: Bioavailability and metabolism Polyamine concentrations in mammals are determined by their nutritional supply, synthesis by the intestinal microbiota, uptake, cellular biosynthesis, catabolism, and urinary excretion. Tissue spermidine concentrations decline with age in model organisms as well as in humans (24–27). This may, at least in part, result from a decline in the biosynthetic activities of polyamine-producing enzymes [reviewed in (28)]. Polyamine metabolism and transport The intracellular spermidine content is the final result of polyamine uptake from the extracellular space, endogenous biosynthesis, catabolism, and excretion (outlined in Fig. 1). Biosynthesis is achieved from the precursor ornithine forming putrescine, spermidine, and spermine (Fig. 1) in tightly regulated step-wise reactions (20, 22, 29). Catabolism of polyamines involves, on the one hand, the oxidative degradation of spermine to spermidine. On the other hand, the degradation and secretion of both spermidine and spermine requires their acetyl-CoA–dependent acetylation by spermine-/spermidine-N1-acetyltransferase 1 (SSAT1) and subsequent oxidation (Fig. 1) (30, 31).
In a recent study, histone deacetylase 10 (HDAC10), a key regulator of autophagy and cell survival (32), has been proposed as yet another mediator of polyamines metabolism owing to its ability to deacetylate spermidine (33). Polyamines also interconnect with diseaserelevant amino acid metabolism. Synthesis of spermidine and spermine requires the formation of decarboxylated S-adenosyl methionine (dcSAM) from SAM. SAM serves as an important cofactor for methylation of proteins, including histones, as well as of DNA, both of which are necessary for the epigenetic control of gene regulation (Fig. 1). Through the putrescine precursor ornithine, polyamine biosynthesis affects the bioavailability of arginine, which is important for the production of nitric oxide (NO), an important signaling molecule that mediates vasodilation, protects from maladaptive cardiac remodeling, affects mitochondrial biogenesis and function, and has vast immunomodulatory effects (34). The transport of polyamines in mammals is less well understood but may involve plasma membrane transporters as present in yeast and bacteria (35). Cellular polyamine uptake and secretion may also be mediated via endocytosis and exocytosis, respectively (Fig. 1). In support of this notion, a vesicular polyamine transporter (VPAT) was identified in astrocytes (36) and mast cells (37 ) that may have important neuro- and immune-modulatory implications (37, 38). Polyamine uptake and excretion In addition to cellular biosynthesis, two other sources are equally important for the systemic availability of spermidine. These sources are (i) external (oral) uptake with the food and (ii) production by intestinal microorganisms (Fig. 2).
Selected unprocessed plant-derived food items are naturally enriched in polyamines, as exemplified by the Durian fruit. Moreover, fermentation processes involving bacteria and fungi used in the food industry cause microbial generation of polyamines, which may contribute to the sometimes subjectively malodorous properties of milk and soy products such as mature cheese and natto, respectively. Ingested spermine and spermidine are quickly adsorbed from the intestinal gut and distributed without degradation (39). Thus, diet influences the blood polyamine concentration, which is highly diverse in humans (40). The average daily nutritional intake of spermidine varies from ~7 to 25 mg and more, with the highest amounts in the Mediterranean diet, which is often described to improve health in humans (41, 42). These values still lie below the amount of spermidine, when calculated based on the optimal food composition proposed by the Swedish Nutrition Recommendations Objectified (43). In mice, oral supplementation of spermidine increases amounts in whole blood (44), serum (45), and tissues (44). Supplementation of the polyamine precursor arginine (alone or in combination with probiotics) may also suffice to increase spermidine concentrations in the colon and blood (46, 47). Indeed, the intestinal luminal concentration of spermidine critically depends on colonic microbiota in mice (48), and this may hold true for humans as well (49).
Together, these studies outline the importance of dietary polyamines and polyamine-producing bacteria for the bioavailability of spermidine (Fig. 2). Given the high content of polyamines in certain types of food, it appears plausible that a polyaminerich diet could overcome the age-associated decline of polyamines. Indeed, daily intake of 50 to 100 g of natto over a 2-month period significantly increased the whole-blood spermine content of healthy human volunteers (40). A cross-sectional observation in central Italy revealed that wholeblood spermidine and spermine content were decreased in elderly humans but remained at the levels of younger (middle-aged) individuals in healthy nonagenarians and centenarians (25). Further studies are warranted to address whether dietary or genetic factors explain the high spermidine content in healthy centenarians and whether they represent a cause or a consequence of healthy aging. Systemic concentrations of polyamines are also controlled through renal secretion. Thus, putrescine and acrolein—a toxic product and by-product, respectively, of spermidine and spermine catabolism by serum amine oxidase—increase in the plasma of patients with chronic renal failure (50). Therefore, dietary supplementation of spermidine in such patients should be carefully reviewed for potential adverse effects. Health effects of dietary spermidine Beyond their capacity to improve cellular fitness— as documented in yeast, plants, and in cultured mammalian cells (table S2)—polyamines (in particular, spermidine) increase life span of multicellular organisms, including nematodes, flies, and mice.
Cardiovascular disease and cancer are the two major causes of death in humans and mice. Similarly to CR regimens, which partially protect against these pathologies, spermidine can delay their manifestation and reduce their severity in rodent models. Life-span extension by spermidine Health- and life-span–promoting effects are documented for dietary or otherwise externally supplied spermidine (Fig. 3A). Spermidine supplementation confers life-span extension both to invertebrate model organisms (19, 24) and to mice (44, 51). Moreover, a diet rich in polyamines reduces mortality of aged mice (52). As true for many other CRMs, spermidine extends life span in a sex-independent manner (table S1). Supplementation of polyamine-producing probiotic bifidobacteria—alone or, moreso, in combination with the polyamine precursor arginine—increases blood concentrations of spermidine and spermine and decreases mortality and the prevalence of cutaneous pathologies in old mice (46, 47). Furthermore, oral spermidine administration counteracts the age-associated disruption of circadian rhythm in mice (45). Spermidine may also ameliorate menopausal decline. Indeed, bone loss induced by ovariectomy in mice, a model of postmenopausal osteoporosis, is prevented with oral spermidine supplementation via inhibition of the formation of (bone-resorbing) osteoclasts (53). The total amount of food polyamines significantly correlates with human life expectancies across distinct Asian countries (54), although this epidemiological study did not adjust for confounding factors typically associated with longevity.
Future well-controlled epidemiological studies are warranted to explore the effects of dietary spermidine on human health span and life span. Polyamine effects on tumorigenesis Polyamines are essential for cell proliferation and growth, and dysregulation of polyamine metabolism is a defining signature of many tumor types; increased polyamine concentrations caused by enhanced biosynthesis can be found in skin, breast, colon, lung, and prostate cancers (35). Given the proliferation-enhancing and cytoprotective effects of polyamines on cultured human cancer cells or xenografted human tumors evolving in immunodeficient mice [reviewed in (55)], polyamines might have procarcinogenic properties. Efforts have been made to suppress polyamine biosynthesis by inhibiting ornithine decarboxylase (ODC) with difluorome thylornithine (DFMO) for the treatment of established cancers in mice, but clinical trials using DFMO have been abandoned because of its toxicity (35). Potentially less toxic, a competitive inhibitor of AdoMetDC (SAM486A), leading to low spermidine concentrations, has been tested in clinical trials for various tumor types, with moderate success in non-Hodgkin’s lymphoma (35, 56). In contrast to potential procarcinogenic properties of polyamines, spermidine supplementation can reduce tumorigenesis in mice. Oral supplementation of probiotic polyamine-producing bacteria (Bifidobacterium animalis subsp.
Lactis LKM512) lowers the incidence of visible skin tumors in aging Crj:CD-1 female mice (47). Dietary spermidine reduces the severity of liver fibrosis and the incidence of hepatocellular carcinomas induced by chemical insults in mice (51). Spermidine administration also slows the growth of CT26 colorectal tumors transplanted into immunocompetent mice (57). Increased intake of dietary polyamines (using chow enriched in spermidine, spermine, and putrescine) causes a delay in chemically induced tumorigenesis in young BALB/c male mice, although the maximum size of tumors increased (58). Thus, in mice polyamines may inhibit colon carcinogenesis yet favor tumor growth, once a cancer has developed. In humans, one study suggested that dietary intake of polyamines above the median could be associated with an elevated risk of developing colorectal adenoma, particularly in women (59). Nevertheless, a subsequent prospective study performed by the same authors failed to confirm this positive association and rather revealed an inverse correlation of high polyamine intake with the risk of colorectal cancers, at least in overweight women with a body mass index (BMI) ≤ 25 (60). Interventional studies are needed to explore the possible effect of dietary spermidine on cancer risk. Spermidine supplementation reduces the growth of transplantable tumors in mice treated with chemotherapies. This spermidine effect is shared by other CRMs (61) as well as by fasting or hypocaloric diets (62, 63) and is mediated by the stimulation of immunosurveillance.
Thus, spermidine and other CRMs enhance the anticancer immune response as they deplete immunosuppressive cells such as regulatory T lymphocytes (Treg cells) from the tumor bed (61). Tumors growing in mice that lack cytotoxic T lymphocytes fail to reduce their growth in response to spermidine or other CRMs (61). Beyond its effects on adaptive immunity, spermidine synthase (SRM)–mediated spermidine production appears to be a determinant for antitumor actions of tumor-associated macrophages (TAMs) in the context of colorectal cancer progression (57). These findings may explain why external supply of spermidine has chemopreventive effects in vivo (that are likely mediated by immunostimulatory effects), although it enhances the proliferation of cancer cells in vitro. Future studies should address associations of dietary spermidine with other types of cancer, especially in light of the potential requirement of polyamines for cancer cell growth. Whether a diet low in polyamines slows growth of specific cancers and whether a polyamine- or specifically spermidine-rich diet would accelerate tumor growth in human patients remain open questions, requiring in-depth investigation before such diets can be broadly recommended, at least for this specific risk group.
Spermidine in cardiovascular and muscle-related disease
Dietary spermidine protects from cardiac aging. It improves diastolic function, left ventricular elasticity, and mitochondrial function in old mice (44). First results from epidemiological studies corroborate these findings in humans: Intake of dietary spermidine (or that of spermidine and spermine combined) inversely correlates with the incidence of cardiovascular disease (CVD) and death in the Bruneck cohort (44). A crosssectional regression meta-analysis of nutritional polyamine content with CVD-caused mortality rates by using publicly available data from 48 Western countries identified negative associations of spermidine and spermine with CVD (64). Spermidine reversed age-induced arterial stiffness with a reduction in oxidative damage of endothelial cells in old mice (65) and alleviated the formation of atherosclerotic plaques in apolipoprotein E–deficient (ApoE−/− ) mice fed a high-fat diet (HFD) for 20 weeks (66). In Dahl salt-sensitive rats fed a high-salt diet (a model of hypertensive heart failure), oral supplementation of spermidine reduced high blood pressure and delayed the transition to heart failure (44), further documenting the antihypertensive (67) and vascular health–promoting (65, 66) functions of dietary spermidine. Humans belonging to the higher tertile of dietary spermidine uptake in the Bruneck cohort had lower diastolic and systolic blood pressure as compared with those of the lower tertile subjects (44)