Effects of spermidine supplementation on cognition and biomarkers in older adults with subjective cognitive decline (SmartAge)—study protocol for a randomized controlled trial
We plan to recruit 100 cognitively normal older individuals with SCD from memory clinics, neurologists and general practitioners in private practice, and the general population. Participants will be allocated to one of the two study arms using blockwise randomization stratified by age and sex with a 1:1 allocation ratio. The primary outcome is the change in memory performance between baseline and post-intervention visits (12 months after baseline). Secondary outcomes include the change in memory performance from baseline to follow-up assessment (18 months after baseline), as well as changes in neurocognitive, behavioral, and physiological parameters (including blood and neuroimaging biomarkers), assessed at baseline and post-intervention.
Background
Longer life expectancy has led to a growth of the older
population, with individuals aged 60 and older expected
to reach about 20% of the population in high-income
countries [1]. This demographic change is associated
with increased rates of age-related diseases, such as dementia due to Alzheimer’s disease (AD). The most
prevalent form of late-onset dementia is expected to
triple by the year 2050 [2], which will pose a high social
and economic impact on patients, caregivers, and society
in general. Importantly, around one third of these cases
worldwide are attributed to potentially modifiable risk
factors [3]. The development of effective strategies that
help to prevent age- and disease-related worsening of
brain structure and function may thus provide significant benefits for society and health-care systems.
We plan to recruit 100 cognitively normal older individuals with SCD from memory clinics, neurologists and general practitioners in private practice, and the general population. Participants will be allocated to one of the two study arms using blockwise randomization stratified by age and sex with a 1:1 allocation ratio. The primary outcome is the change in memory performance between baseline and post-intervention visits (12 months after baseline). Secondary outcomes include the change in memory performance from baseline to follow-up assessment (18 months after baseline), as well as changes in neurocognitive, behavioral, and physiological parameters (including blood and neuroimaging biomarkers), assessed at baseline and post-intervention.
Background
Longer life expectancy has led to a growth of the older
population, with individuals aged 60 and older expected
to reach about 20% of the population in high-income
countries [1]. This demographic change is associated
with increased rates of age-related diseases, such as dementia due to Alzheimer’s disease (AD). The most
prevalent form of late-onset dementia is expected to
triple by the year 2050 [2], which will pose a high social
and economic impact on patients, caregivers, and society
in general. Importantly, around one third of these cases
worldwide are attributed to potentially modifiable risk
factors [3]. The development of effective strategies that
help to prevent age- and disease-related worsening of
brain structure and function may thus provide significant benefits for society and health-care systems.
Subjective cognitive decline: a target group for early intervention
Subtle neuropathological alterations related to AD are suggested to start decades before the onset of clinical symptoms [4, 5]. One early sign of pathological brain aging is the manifestation of subjective cognitive decline (SCD). In cognitively unimpaired older individuals, the presence of SCD is associated with a higher risk for objective cognitive decline and clinical progression to symptomatic disease stages [6–9]. Moreover, individuals with SCD harbor increased β-amyloid (Aβ) deposition [10], gray matter volume reduction [11–13], and neural dysfunction [14] in brain regions typically affected in AD. Thus, SCD has been conceptualized to occur at a late preclinical stage of AD [15], where aberrant brain changes are present [16] in the absence of objective cognitive impairment. This at-risk group is recognized as an eligible target population for early intervention strategies [15, 17], aiming to protect against neuropathological alterations, to restore functional and structural brain health, and to maintain cognitive abilities as long as possible.
Spermidine and its implication in healthy aging
protect against age- and disease-related brain changes and, thereby, preserve cognitive functioning [18–22]. Caloric restriction, among others, appears to be effective to improve memory performance in the elderly [23] and induce favorable neural changes in the hippocampal network [24]. Novel candidate substances proposed to mimic such beneficial effects in aging organisms are natural polyamines, in particular, spermidine and spermine [25, 26].
Organic compounds play an important role in the maintenance of basic cellular functions like cell growth, survival, and proliferation [27]. Beyond these “microscopic” actions, there is an indication that polyamines influence “macroscopic” systems underlying learning and memory as well as age-related changes of this cognitive function. In rodent models, polyamine levels in the hippocampus are shown to be associated with memory retrieval and formation [28] and change with age in certain brain areas, including medial-temporal memory structures [29]. Lastly, the reduction of spermidine in the brain of aged flies is paralleled by memory decline [30]. Given these observational findings, it has been hypothesized that external supply of polyamines may protect against age-related memory loss. Indeed, a first study in aging fruit flies showed that spermidine-rich diet restored endogenous spermidine levels and thereby rescued memory performance [30]. This beneficial effect of spermidine intake appears to be mediated by several protective pathways [31]. For example, spermidine may act through autophagy to regulate synaptic transmission/plasticity [32] and clear cellular “waste” including pathogenic protein aggregates [30]. Nutritional spermidine is also associated with a number of cardio-protective [33] and anti-inflammatory [34] actions, which may help to preserve higher-order brain functions. Overall, these findings in aging model organisms have suggested a promising role of spermidine in the promotion of brain and cognitive health.
Spermidine supplementation in older adults with SCD: initial evidence
Spermidine supplementation is thus proposed to open a new avenue in the protection and restoration of memory abilities in higher age.
This expected benefit is of particular need in older individuals at risk for the development of dementia. It is, however, unknown whether the memory-promoting effect of spermidine is detectable in humans and to what extent this effect may be attributed to the influence of spermidine on biomarkers of healthy aging. An initial study showed that polyamine-enriched diet over 2 months increased blood spermidine levels in healthy middle-aged men [35]. Our group has conducted a first 3-month phase IIa trial with nutritional spermidine in 30 older adults with SCD (ClinicalTrials.gov identifier NCT02755246). Trial outcomes demonstrated high compliance, tolerance, and safety profiles as well as preliminary efficacy of the administered spermidine-rich plant extract [36, 37]. Specifically, we found a moderate enhancement of memory performance, measured using the mnemonic similarity task (MST) [38], in the spermidine-treated group compared with placebo intervention. This computer-based task is a sensitive measure of subtle cognitive changes induced by targeted interventions in the memory system [39]. In the pilot trial, we did not detect intervention effects on standard neuropsychological tests of memory or executive functions [37]. At this point, longer-term intervention studies with sufficient sample size are required to validate the therapeutic potential of nutritional spermidine against memory loss in older individuals and delineate possible neurophysiological mechanisms of action.
Choice of comparator
A placebo comparator will be implemented in this study, in correspondence with our previous trial [36]. The present trial will use microcrystalline cellulose as a comparator condition. Beside randomization and double blinding, placebo-controlled trials allow to minimize the risk of bias and to maximize the verification of the effect of the verum intervention [40]. Our placebo capsules will be identical to the verum intervention in shape, color, taste, and smell, but contain no active ingredients. The World Health Organization (WHO) and the US Food and Drug Administration (FDA) recognize that the use of cellulose as a food additive is safe and well tolerated in animal models and humans.
Objective and purpose of the SmartAge trial
We will conduct a randomized controlled trial with a 12-month spermidine supplementation in cognitively unimpaired older individuals with SCD (n = 100). The primary objective of the SmartAge trial is to provide evidence of a beneficial impact of nutritional spermidine on memory performance (primary outcome) at the end of the intervention, as compared with placebo. Second, we aim to examine whether spermidine intake has positive effects on memory performance after an additional 6-month follow-up period without further supplementation as well as on other age-relevant cognitive domains, lifestyle behaviors, psycho-affective characteristics, and perceived quality of life.
This data will help to estimate potential benefits of nutrition intervention on well-being and everyday life. Third, this trial will identify possible mechanisms of action underlying the proposed spermidine-associated benefits on cognition using indicators of autophagy, blood-based biomarkers, and neuroimaging parameters of brain structure and function. Finally, the study will assess potential moderators of the intervention effect, such as age-related neuropathologies as well as genetic polymorphisms. Overall, the SmartAge trial aims to establish a significant milestone in the implementation of early intervention strategies in older individuals at risk of dementia due to AD.
Methods: participants, intervention, and outcomes
Trial design and setting
This is a monocentric, randomized, double-blind, placebo-controlled phase IIb trial, carried out at the NeuroCure Clinical Research Center, Charité – Universitätsmedizin Berlin. The trial includes 12 months of intervention with spermidine supplementation (target intervention) compared with 12 months of placebo intake (control intervention). The trial will compare outcomes of the two intervention groups, with participants randomized to one of the two study arms. Randomization is performed blockwise with a 1:1 allocation ratio. The SmartAge trial has been approved by the responsible Institutional Review Board and will be carried out in compliance with institutional ethical standards and the Declaration of Helsinki.
Eligibility criteria
The main inclusion criteria for potential participants in the SmartAge study are:
1. Age, 60–90 years 2. Presence of SCD in accordance with research criteria, recommended by the international SCD-I working group for studies on SCD [15]: the expression of subjective cognitive complaints for at least 6 months and associated concerns (worries), affirmation to consult and/or previous consultation of a doctor due to these symptoms, normal cognitive performance, and no restrictions on activities of daily living 3. Ability to provide written informed consent 4. Health insurance coverage to clarify possible incidental findings We will exclude potential participants in case one or more of the following criteria are present: 1. Dementia, according to the Diagnostic and Statistical Manual of Mental Disorders, 4th Edition (DSM-IV) [41] 2. Mild cognitive impairment (MCI), according to clinical diagnostic criteria [42] 3. Severe or untreated medical disorders (advanced cardiac or respiratory disease, severe liver, kidney or metabolic diseases, untreated thyroid dysfunctions or untreated diabetes mellitus), psychiatric disorders (untreated depression, psychosis) or neurological disorders (epilepsy, clinically manifest stroke) 4. Malignancies currently or as indicated by medical history (exception: basalioma) 5. Drug abuse or alcohol dependency 6. Current polyamine substitution and/or participation in respective intervention studies 7. Known intolerance or allergies to wheat germs, gluten or histamine 8. Contraindications to imaging techniques: claustrophobia, metallic implants (e.g., intracranial metal clips), electronic devices (e.g., cardiac pacemakers), or permanent tattoos
9. With regard to positron emission tomography (PET) assessments (optional consent): participation in another study with the use of ionizing radiation within the last 3 months 10. With regard to muscle biopsy assessments (optional consent): allergy or intolerance to the local anesthetic (lidocaine), coagulation disorders, current therapy with antiplatelet or anticoagulation drugs (e.g., clopidogrel, aspirin, vitamin K antagonist, new oral anticoagulant drugs) or steroids, current thrombosis, or other severe diseases of the lower extremities that precludes muscle biopsy In case written informed consent is provided and all eligibility criteria are met, participants will be included in the study.
Intervention
Participants will receive nutrition intervention over 12 months with either spermidine or placebo supplementation. The dietary supplement used in the target intervention is a polyamine-rich plant extract [36], produced using an extraction method developed and optimized by TLL The Longevity Labs (Graz, Austria). The extraction method will obtain polyamines from wheat germs without the application of acids, organic solvents, and/or potentially harmful chemicals. Wheat germs serve as raw material for the extraction process, because they contain a high concentration of polyamines, in particular, spermidine [43]. The plant extract to be administered is mainly enriched by spermidine and spermine (with 1.2 mg spermidine and 0.6 mg spermine per 1 g extract). Furthermore, 1 g of extract contains 0.2 mg putrescine, < 0.005 mg cadaverine, and 0.166 mg L-ornithine.
In combination with a normal diet, the intake of the planned daily dose of 750 mg extract (even in the case of multiple overdoses) is below the calculated no observed adverse effects level (NOAEL) for humans of 29 mg/kg body weight (bw)/day for cadaverine and putrescine [44]. This is also the case for spermidine and spermine, where the NOAEL is 13.5 mg/kg bw/day or 3.1 mg/kg bw/day [44]. The NOAEL for L-ornithine in humans is above 500 mg/ kg bw/day [45], and thus remains unattainable in the planned extract administration. The safety of the spermidine-rich extract for the use in humans was evaluated prior to the SmartAge trial by a chemical analysis (unpublished data) and a translational study on safety and tolerability [36]. In the intervention group, the spermidine supplement will be administered daily in the form of six capsules, each containing 125 mg extract, resulting in a daily dose of 750 mg extract or 0.9 mg spermidine, 0.5 mg spermine, 0.2 mg putrescine, < 0.004 mg of cadaverine, and 0.12 mg of L-ornithine. This amount of daily polyamine intake accounts for an increase of approximately 10– 20% of the average spermidine intake in industrial countries [46]. The dosage is within the amount of polyamines administered in an earlier intervention study [35], using approximately 10 mg/day of dietary spermidine in humans. The amount would also be obtainable by well-targeted diets (e.g., 200 g of cooked soybeans [47]).
The control group will receive placebo capsules, six per day, filled with 750 mg cellulose in sum. Participants of both groups (spermidine and placebo) are instructed to follow a regular capsule intake per day, two capsules with each main meal (breakfast, lunch, dinner), and to maintain their dietary habits during the time of intervention. Participants will be supplied with capsules throughout the intervention period. To ensure trial compliance, we closely monitor capsule intake throughout the trial (see the “Intervention period” section).
Assessment of study measures
Measures assessed at baseline
Assessments of following participant’s characteristics will be conducted at baseline, summarized in Table 1: (a) demographic information including age, civil status, and education; (b) information on family history focused on AD, other non-specified subtypes of dementia, idiopathic Parkinson’s disease, and stroke; and (c) behavioral measures of subjective cognition function, lifelong experience, and personality traits. In addition, (d) physiological measures of brain Aβ status, measured using [18F] florbetaben (FBB) PET, and genotype information on apolipoprotein E (APOE) ε4 status along with other learning-relevant polymorphisms, measured using genotyping of blood-derived deoxyribonucleic acid (DNA), will be obtained. Note that potential changes in demographic information and family history will be recorded throughout the trial.
Outcome measures
Assessments of study outcomes will be conducted at the baseline visit and post-intervention visit (12 months after baseline). Selected outcome measures will be assessed at the follow-up visit (18 months after baseline). Primary and secondary outcomes are summarized in Table 1. Study outcomes will be collected in accordance with standardized operational procedures (SOPs) and study assessors that are blinded to the type of intervention. Primary endpoint: The primary endpoint of this trial is the change in memory performance between baseline visit (V1) and post-intervention visit (V2). Memory performance is operationalized by mnemonic discrimination ability, to be assessed by the MST [38]. Mnemonic discrimination performance is evaluated due to its established sensitivity and robustness to memory deficits associated with aging and neurodegenerative disease [48–50]. Moreover, this behavioral marker is closely tied to neural dysfunction in the hippocampal memory network [48, 49] and has been identified as a sensitive outcome measure in older individuals at higher risk of AD [39]. The visual memory task is available for public download (http://faculty.sites.uci.edu/starklab/mnemonic-similaritytask-mst/) with multilingual instructions. Sets of well-matched stimuli will be presented at baseline, post-intervention, and follow-up visits respectively, with a pseudorandomized order of stimuli within each set. The MST consists of two phases: During the incidental encoding phase, participants view images of everyday objects and decide on each trial, whether the object is typically found “outdoors” or “indoors.”
During the subsequent recognition phase, images are repeated (repetition items), randomly inter-mixed with novel images (foil items), and images that are perceptually similar to those pictures seen during the encoding phase (lure items). Participants will be asked to indicate for each trial, whether the image was “old”, “new” or “similar”. From the proportion of responses provided during recognition, a response bias-corrected mnemonic discrimination index will be calculated, similar to previous reports [37, 38, 48]. Secondary endpoints: Secondary outcomes include the change in memory performance (operationalized by mnemonic discrimination performance) between baseline visit (V1) and follow-up assessment (V3, 18 months after baseline). Additional secondary endpoints (see Table 1) are changes in the following outcome measures: (a) Neuropsychological parameters on verbal and visualspatial memory, attention, executive functions, and sensorimotor speed, assessed at V1, V2, and V3 (b) Behavioral parameters of lifestyle behaviors, psycho-affective characterization and perceived quality of life, assessed at V1, V2, and V3, as well as (c) Physiological parameters including autophagy signaling (measured in muscle biopsies), peripheral vascular parameters (measured in blood), and parameters of brain structure, perfusion, and function (measured using cerebral magnetic resonance imaging [MRI]) to be assessed at V1 and V2.
Finally, exploratory outcomes (see Table 1) of subjective cognitive function, cardiovascular risk factors, as well as muscle function and strength markers (available for a sub-sample) will be evaluated. Using moderator analysis, we will further assess whether demographic factors (including sex), genetic phenotype (including APOE), and presence of brain pathology (including a positive brain Aβ status) affect outcomes of the intervention.
Participant timeline
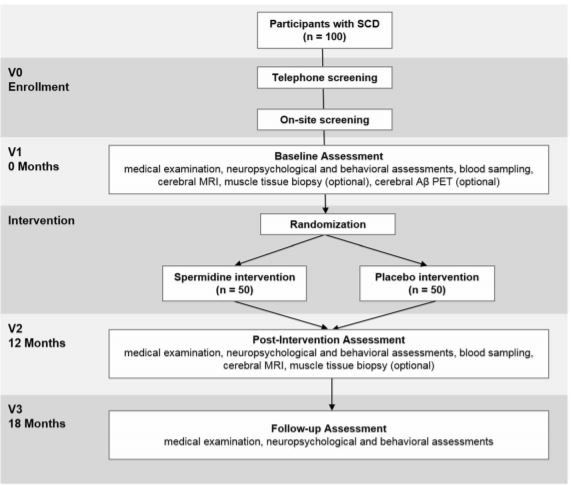
The SmartAge trial will involve five phases for each participant: study enrollment, which included screening assessment (V0), a baseline visit (V1), a 12-month intervention period, a post-intervention visit (V2), and a follow-up visit (V3). Trial phases are described below and summarized in Fig. 1.
Enrollment (V0)
Individuals, who express interest in study participation, will undergo following screening procedure to ensure study eligibility. A standardized pre-screening interview conducted over the phone will be administered to collect information on medical and demographic data, on MRI/PET suitability, and on suitability for muscle biopsy. During this interview, the following questions will be asked to endorse the presence of SCD with associated concerns (worries), similar to previous reports and recommendations [15, 51]. Question 1 will be “Do you feel that your cognitive performance has become worse?” (German: “Haben Sie das Gefühl, dass Ihre geistige Leistungsfähigkeit schlechter geworden ist?”, possible answers: yes/no).
In case of a positive answer to question 1, question 2 will be asked: “Since when do you have the feeling that your cognitive performance has gotten worse?” (German: “Seit wann hat sich ihre geistige Leistungsfähigkeit verschlechtert?”). In case the answer to question 2 is at least 6 months, question 3 will be asked: “Are you concerned about this cognitive worsening?”(German: “Bereitet Ihnen diese Verschlechterung Sorgen?”, possible answers: yes/no). In case of a positive answer to question 3, question 4 will be asked: “Would you seek or have you sought medical help due to this cognitive worsening?” (German: “Würden Sie diesbezüglich einen Arzt aufsuchen bzw. haben Sie dies bereits getan?”, possible answers: yes/no). Affirmative responses to diagnostic questions 1, 3, 4, and a duration of SCD for at least 6 months are mandatory for study inclusion. Potential participants that meet inclusion criteria as evaluated during pre-screening will be invited for the on-site screening visit to the Charité – Universitätsmedizin Berlin. Each individual will receive a standardized screening assessment, including neuropsychological tests and questionnaires, to certify the absence of objective cognitive impairment and current psychiatric disorder (e.g., depression). Following measures will be used: (a) Mini-Mental State Examination (MMSE) [52] score ≥ 26, (b) performance above − 1.5 standard deviation (SD) of age-adjusted norms in the Logical Memory II subscale total delayed recall (Story A and B) [53], and the Trail Making Test (TMT) A [54], (c) no deficits on selected items of the Instrumental Activities of Daily Living Scale (IADL) [55], and (d) a Geriatric Depression Scale (GDS) [56] score ≤ 10.
After successful completion of the on-site screening assessment, eligible participants will proceed to baseline assessment.
Baseline visit (V1)
The baseline visit will encompass a maximum of 4 days with different assessments conducted on each day. On day 1 (carried out immediately after screening, duration: approx. 5 h with breaks), each participant will receive a standardized medical examination that includes fasting blood sampling and physical assessments. Next, a standardized neuropsychological test battery and a questionnaire battery will be administered (Table 1). On day 2, each participant will undergo the MRI assessment (duration: approx. 2 h). On day 3, muscle tissue biopsy will be carried out to determine autophagy signaling (optional consent, duration: approx. 2–3 h, aiming for 25 participants). On day 4, a cerebral Aβ PET assessment is performed (optional consent, baseline visit only, duration: approx. 2–3 h, aiming for 50 participants). After completion of the baseline visit, participants will be randomly assigned to one intervention arm. The Aβ PET measurements will in most cases be conducted after intervention has started, due to logistic reasons.
Intervention period
During the intervention, at 3 months, 6 months, and 9 months after the baseline visit (V1), telephone or on-site interviews will be scheduled with each participant to obtain information on participants’ physical and mental well-being, trial compliance, changes in dietary habits, and current medication intake.